Table of Contents
In advanced medicine, the distinction between autologous and allogeneic sources is foundational—not just in clinical care, but throughout drug development and pharmaceutical manufacturing. While most commonly referenced in cell and gene therapy, these concepts impact a broad array of biologic products, blood-based medicines, and tissue-derived therapies. For contract development and manufacturing organizations (CDMOs) such as Adragos Pharma, understanding these terms is critical to supporting client innovations from bench to patient.
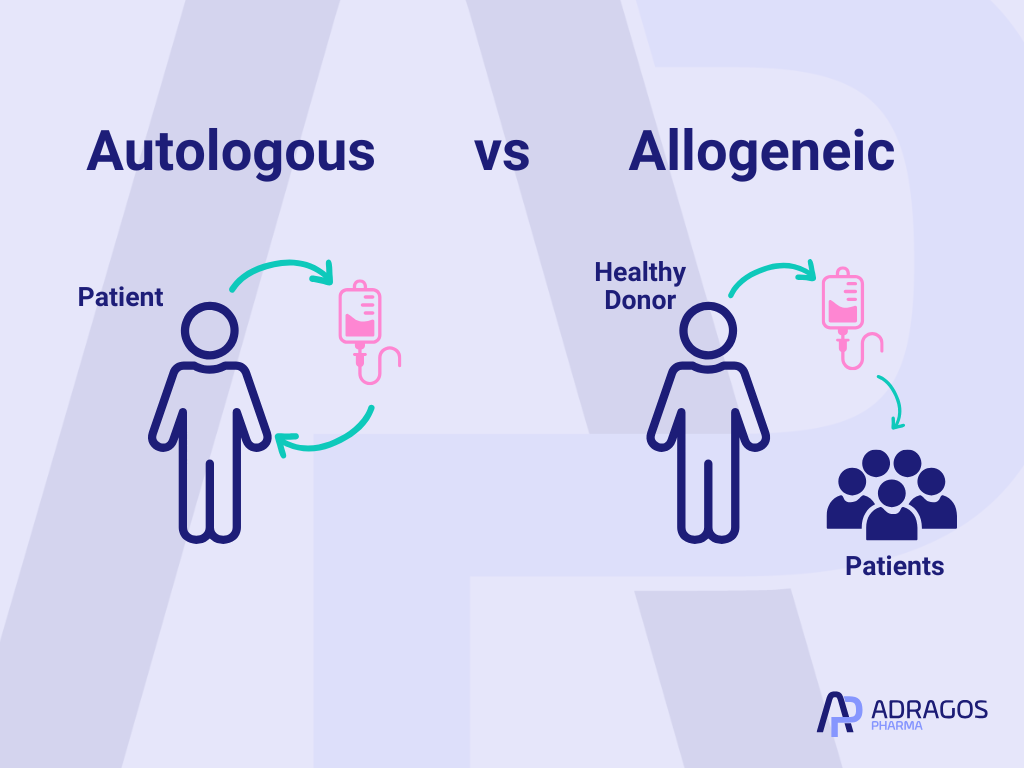
Decoding the Basics: What Are Autologous and Allogeneic Approaches?
Simply put, autologous means “from yourself—back to yourself.” When a treatment uses cells, tissues, or biological materials collected from a patient and then gives them back after processing, that process is called autologous. This option offers a high level of personalization and is typically less likely to trigger the body’s defense systems.
On the other hand, allogeneic means “from a donor to a recipient.” Here, materials come from another person (often carefully matched and screened). This approach is fundamental for creating standardized, “off-the-shelf” therapies that can serve many patients with a single batch.
Both meet unique and rapidly growing needs in pharmaceutical development—but they also come with their own set of challenges.
Common Therapeutic Uses of Autologous and Allogeneic
Biologics & Plasma Therapies
- Autologous Examples: Think of niche treatments like platelet-rich plasma (PRP) therapy, where a patient’s own blood is processed and returned for wound healing or joint regeneration.
- Allogeneic Examples: Most blood plasma products on the market—like immunoglobulins or clotting factors—are pooled from several healthy donors, purified, and delivered to patients in need worldwide.
Tissue Engineering and Implants
- Autologous Grafts: Here, a patient may have a piece of bone, skin, or tendon removed, treated, and re-implanted in another part of their body. This approach significantly reduces the risk of rejection or complications.
- Allogeneic Grafts: Alternatively, donor tissue (skin, bone, valves) from tissue banks is standardized and used in reconstructive surgeries, trauma care, or transplants for patients lacking healthy autologous material.
Next-Generation Vaccines and Personalized Medicine
- Autologous Vaccines: The future holds vaccines made from a patient’s own tumor antigens, potentially leading to hyper-targeted cancer therapies.
- Allogeneic Vaccines: Everyday flu shots or COVID-19 vaccines are allogeneic—engineered for the masses, with broad spectrum effectiveness.
Advantages and Disadvantages: Autologous vs Allogeneic
Autologous Approaches
Advantages:
- Unmatched compatibility: Since it’s your own material, risk of immune rejection or graft-versus-host disease is minimal.
- No long wait times: No need to search for a matching donor—everything starts with you.
Disadvantages:
- Personalized means complex: Every treatment batch is unique to each patient, requiring meticulous tracking and customization.
- Time & scalability constraints: This process can be slower and may not be ideal for large urgent patient populations.
Allogeneic Approaches
Advantages:
- Wider access: One donor’s contribution can help dozens (or hundreds) of recipients.
- Faster response: Standardized batches mean therapies can be made in advance and ready as soon as they’re needed.
Disadvantages:
- Immune risks present: Even with careful matching, there’s still a potential for immune response or rejection.
- Safety and screening: Rigorous donor screening and regulatory controls are always essential.
A Partner for Flexible and High-Quality Manufacturing
Whether a pharmaceutical program relies on autologous or allogeneic principles, the path from development to delivery requires adaptable, high-quality manufacturing. For projects demanding agility—such as small-batch, patient-specific runs—or those requiring the highest compliance for broader-scale, standardized production, robust expertise and advanced infrastructure are essential.
With over 20 years of experience, Adragos Pharma’s Swiss Jura facility offers comprehensive support tailored to both clinical and commercial manufacturing. Specializing in the aseptic production of lyophilized and liquid sterile (bio)pharmaceutical products through fill and finish, the facility is ideally suited for small clinical and commercial batches. Certified by both GMP and FDA, Jura upholds the highest quality and safety standards.
Clients benefit from end-to-end solutions:
- Scale-up and Technology Transfer: Seamless transition from development to production, backed by strong technical expertise.
- Flexible Batch Production: No minimum batch sizes—ensuring the ultimate flexibility for unique, complex, or limited-quantity projects.
- Aseptic Process Simulation (APS): Fully compliant APS services reinforce product sterility and efficacy.
- GMP & FDA Regulatory Support: Comprehensive compliance guidance, making even the most complex clinical projects manageable.
- API Management and Specialized Formulations: Skilled handling of sensitive or valuable materials, including peptides, biologicals, controlled substances, and ophthalmic products.
From pilot and scale-up batches for preclinical and early-phase clinical studies to full clinical or commercial manufacturing, release analytics, packaging, stability studies, and GMP storage, Adragos Jura delivers Swiss precision and reliability every step of the way.
By integrating technology, technical know-how, and proactive project management, the facility empowers clients to confidently advance their products—whether highly-personalized (autologous) or broadly-distributed (allogeneic)—from early development through to market launch.
In conclusion, the division between autologous and allogeneic is about much more than biology—it shapes every stage of the pharmaceutical journey, from research and regulatory planning to manufacturing and logistics. As the world moves toward more personalized and precise therapies, making an informed choice is essential for patients and developers alike.
At Adragos Pharma, we help our partners navigate these complexities, ensuring every product—whether made for one or many—meets the highest standards of safety, efficacy, and quality.
FAQs: Autologous vs Allogeneic
What’s the difference between autologous and allogeneic?
Autologous utilizes the patient’s own cells, minimizing rejection risk; allogeneic uses genetically matched donor cells, requiring tissue compatibility and often delivering immune-mediated therapeutic benefits.
What is the difference between autologous and allogeneic transfusions?
Autologous transfusion involves a patient’s own blood collected ahead of time. Allogeneic transfusion uses blood from another person and is subject to immune matching protocols.
What is an example of allogeneic?
A bone marrow transplant from an unrelated donor for treatment of relapsed leukemia.
What is the purpose of autologous?
To enable safe transplantation and cell therapies without the risk of immune-mediated rejection or graft versus host disease.
Is CAR-T allogeneic or autologous?
Most current CAR-T cell therapies are autologous, using the patient’s own immune cells. Next-generation products are exploring allogeneic CAR-T using healthy donor sources.
What is meant by allogeneic?
A biological product or tissue derived from a genetically different individual of the same species.
What is an autologous transplant?
A therapeutic procedure in which a patient receives an infusion of their previously harvested and stored bone marrow, blood stem cells, or blood after high-dose therapy.
