Drug discovery and development is the cornerstone of pharmaceutical advancements, encompassing the transformation of promising scientific concepts into approved, effective medications. This intricate journey involves multiple stages, each essential in ensuring that new drugs are safe, effective, and ready for market.
With the advancement of artificial intelligence (AI) and machine learning (ML), drug development is becoming faster and more precise, revolutionizing target identification, lead optimization, and high-throughput screening. In this article, we’ll explore the drug development process in detail, offering insights into each phase while emphasizing the importance of scientific research, regulatory compliance, and emerging technologies.
What Is the Drug Development Process?
The drug development process is a series of structured steps designed to convert new drug ideas into marketable therapeutic agents. Pharmaceutical companies play a crucial role in this process, monitoring the safety of their drugs using the FDA Adverse Event Reporting System (FAERS) and post-marketing surveillance studies.
Emerging technologies like AI-driven drug discovery, computer-aided drug design (CADD), and drug delivery innovations are helping accelerate the development of small molecules and biologics for various diseases, including cancer, neurological disorders, and rare diseases.
Phases of the Drug Development Process
The drug development process involves several stages, including drug discovery, preclinical and clinical testing, regulatory approval, and post-marketing surveillance. Each phase is meticulously planned to ensure that only safe and efficacious drugs reach patients.
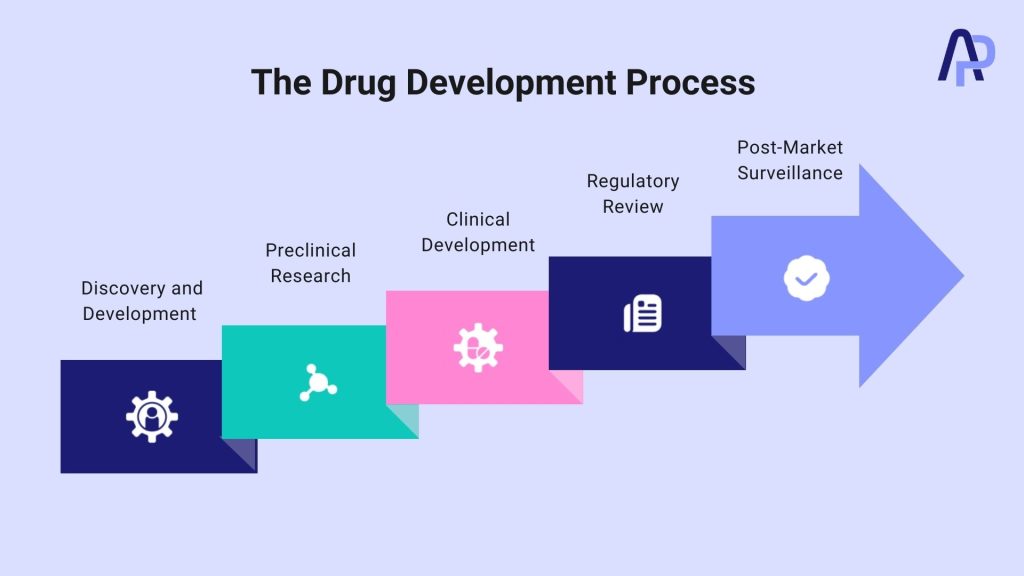
Step 1: Discovery and Development
The journey begins with the identification of potential drug targets. Researchers utilize:
- Artificial intelligence (AI) and machine learning (ML) to analyze large datasets for novel drug candidates.
- High-throughput screening (HTS) to rapidly test thousands of chemical compounds for therapeutic potential.
- Target identification and lead optimization to refine the best compounds for drug development.
💡 AI drug discovery has significantly shortened timelines by predicting molecular interactions and optimizing lead compounds before moving into preclinical testing.
Step 2: Preclinical Research
Before a drug enters human trials, it undergoes rigorous preclinical studies to ensure safety, toxicity, and pharmacokinetics.
Key Aspects of Preclinical Drug Development:
- Animal Models & In Vitro Testing: Evaluating toxicity and therapeutic effects in cell cultures and animal models (e.g., rodents, primates).
- Pharmacokinetics & Pharmacodynamics (PK/PD): Understanding how the drug is absorbed, distributed, metabolized, and excreted (ADME).
- Toxicology Studies: Assessing potential side effects and maximum tolerated dose (MTD).
Once the drug demonstrates safety, researchers file an Investigational New Drug (IND) application with the FDA to seek approval for human trials.
Step 3: Clinical Development
Clinical trials are divided into multiple phases (I-IV), each designed to systematically evaluate a new drug’s safety, efficacy, and optimal dosage.
Phase I: Safety & Dosage in Healthy Volunteers
- Conducted on a small group of healthy participants.
- Determines drug metabolism, bioavailability, and potential side effects.
Phase II: Effectiveness & Safety in Patients
- Involves a larger group of patients with the target disease.
- Assesses therapeutic efficacy and short-term side effects.
Phase III: Large-Scale Trials for Regulatory Approval
- Includes thousands of patients across multiple locations.
- Generates comprehensive data for regulatory review (FDA, EMA, etc.).
💡 AI in clinical trials is helping optimize patient selection and predict trial outcomes, reducing failure rates.
Step 4: Regulatory Review
After successful Phase III trials, a New Drug Application (NDA) or Biologics License Application (BLA) is submitted to regulatory agencies like the:
- U.S. Food and Drug Administration (FDA)
- European Medicines Agency (EMA)
- Other regulatory bodies worldwide
Regulatory agencies assess:
✔ Efficacy & safety data
✔ Risk-benefit analysis
✔ Manufacturing & quality control standards
💡 FDA approval pathways (e.g., Fast Track, Breakthrough Therapy, Priority Review) help accelerate the development of drugs for critical diseases like cancer and rare genetic disorders.
Step 5: Post-Market Surveillance
Phase IV Studies
Once a drug is approved, Phase IV (post-marketing) trials continue to monitor:
- Long-term safety & real-world effectiveness
- Drug interactions & rare adverse events
- Pharmacovigilance studies
The FDA, EMA, and pharmaceutical companies use real-world data (RWD) and artificial intelligence to detect rare side effects.
💡 Post-marketing surveillance is essential for ensuring patient safety, as some side effects only emerge after years of widespread use.
Emerging Trends in Drug Development
The pharmaceutical industry is rapidly evolving with cutting-edge innovations such as:
✅ AI & Machine Learning in Drug Discovery – Identifying novel drug candidates faster.
✅ CAR-T Cell Therapy – Personalized cancer treatments.
✅ Advanced Drug Delivery Technologies – Improving bioavailability and targeted drug delivery.
✅ Computer-Aided Drug Design (CADD) – Enhancing small molecule discovery.
Organizations like the Cancer Drugs Fund (UK) and Oak Foundation are funding high-impact drug development research.
The drug development process is a complex yet rewarding journey that transforms scientific discoveries into life-saving medicines. From target identification and AI-driven drug discovery to clinical trials and post-marketing surveillance, each step ensures drug safety, efficacy, and regulatory compliance.
💡 With AI, machine learning, and real-world evidence collection, the future of pharmaceutical development is set to become more efficient, cost-effective, and patient-centered.
🚀 As innovations like AI drug discovery and precision medicine advance, we can expect faster breakthroughs in treating diseases worldwide.
Key Takeaways
✅ AI & machine learning are transforming drug discovery.
✅ Regulatory agencies (FDA, EMA) play a critical role in drug approval.
✅ Clinical trials (Phases I-IV) are essential for safety & efficacy.
✅ Post-marketing surveillance ensures long-term drug safety.
✅ Drug delivery innovations are enhancing therapeutic outcomes.
FAQs about Drug Development Process
What Is the Pathway of New Drug Development?
The pathway includes drug discovery, preclinical testing, clinical trials, regulatory review, and post-marketing surveillance to ensure the drug’s safety and efficacy.
What is the difference between Phase I, Phase II, and Phase III clinical trials?
Phase I trials focus on safety and dosage with a small group of healthy volunteers or patients. Phase II trials expand the focus to include effectiveness and further safety testing in a larger group of patients. Phase III trials involve large-scale testing to confirm effectiveness, monitor side effects, and compare the drug to commonly used treatments.
How long does the drug development process typically take?
The drug development process can take anywhere from 10 to 15 years, depending on the complexity of the drug and the thoroughness of the testing required.
What factors can lead to drug failure during development?
Drug failure can occur due to lack of efficacy, safety issues discovered during clinical testing, or challenges in the formulation and manufacturing process. Financial constraints and regulatory hurdles may also contribute to drug development failures.
What is the importance of preclinical studies?
Preclinical studies are crucial for assessing the safety and biological activity of a drug in the laboratory and animal models before moving on to human trials. These studies provide a foundation for understanding how the drug works and its potential risks.
