In today’s dynamic pharmaceutical industry, understanding the vast spectrum of CDMO (Contract Development and Manufacturing Organization) services can be crucial for pharmaceutical companies. The landscape of drug development and drug manufacturing is complex and requires an in-depth understanding of various processes and services that these organizations provide. This blog post will delve into CDMO services, elucidating how they can facilitate your pharma journey from drug discovery to commercial production.
What Are CDMO Services?
A CDMO, or Contract Development and Manufacturing Organization, offers comprehensive services that encompass the spectrum of drug development and manufacturing for pharmaceutical companies. These organizations are pivotal in the pharma industry, providing support ranging from initial drug discovery through to large scale commercial manufacturing, including the development and manufacturing of drug substances, ensuring efficiency and compliance at every stage.
Key CDMO Services
A variety of key services offered by CDMOs can significantly enhance your pharmaceutical projects. Here’s a comprehensive list of top solutions provided by leading CDMOs to transform your drug development journey:
CDMOs also offer end-to-end clinical trial capabilities and support, ensuring expertise across the entire supply chain for various types of investigational medicinal products (IMPs).
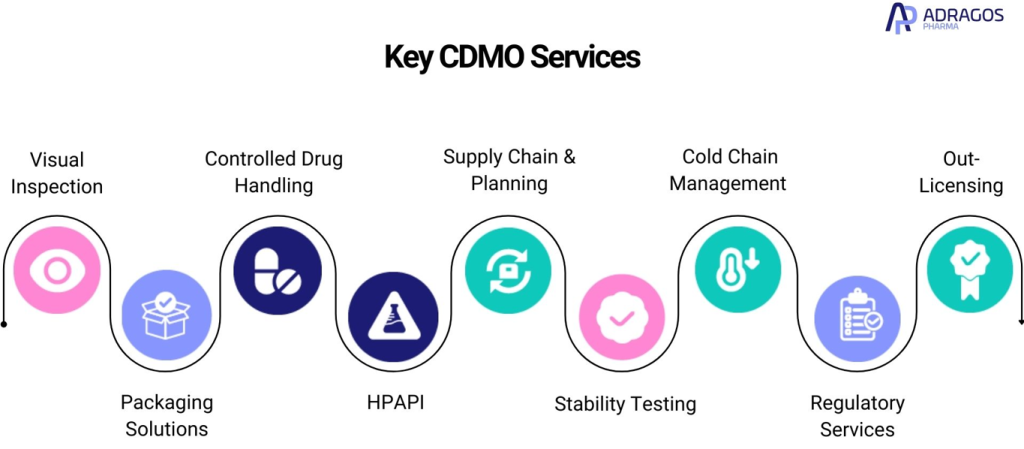
1. Visual Inspection
Visual inspection services from CDMOs ensure that products meet strict regulatory and quality standards. Utilizing advanced technology and cutting-edge equipment for precise and reliable inspections, these services verify the integrity and safety of pharmaceutical products before reaching the market.
2. Pharmaceutical Packaging
Custom packaging solutions provided by CDMOs are designed to meet the specific requirements of different pharmaceutical products. These solutions ensure innovation, product protection, security, and regulatory compliance tailored to diverse product needs.
3. Controlled Drug Handling
Handling controlled substances requires specialized facilities and expertise. CDMOs offer secure environments and knowledgeable staff to manage these high-stakes pharmaceuticals safely.
4. High Potency APIs (HPAPI)
The synthesis of high potency active pharmaceutical ingredients (HPAPIs) is essential for the development of advanced therapies. CDMOs provide state-of-the-art facilities and expert personnel to handle these complex compounds efficiently.
5. Supply Chain Planning
In the pharmaceutical industry, supply chain and planning are critical components that ensure the smooth and timely delivery of products to market. Efficient supply chain management harmonizes all stages of production and distribution, from sourcing raw materials to delivering the final product to consumers
6. Drug Stability Testing
Stability testing services ensure that pharmaceuticals maintain their efficacy and safety throughout their shelf life. CDMOs conduct thorough testing including raw material, in-process, and final product stability testing to comply with regulatory standards and meet market requirements.
7. Cold Chain Management
Maintaining the cold chain is crucial for temperature-sensitive drugs. CDMOs offer reliable cold chain management services to preserve product quality and integrity throughout the distribution process.
8. Regulatory Services
Navigating the regulatory landscape is a significant challenge for pharmaceutical companies. CDMO regulatory services provide comprehensive support, from initial consultation to securing regulatory approvals and post-market compliance.
9. Out Licensing
Out-licensing services enable pharmaceutical companies to monetize non-core assets by transferring the rights to develop and commercialize their proprietary drugs to other organizations. This strategic partnership allows companies to focus on their core competencies while generating revenue from innovative products developed by trusted partners.
The Importance of CDMO Services in Drug Development
Drug Development Process
The drug development process is intricate and lengthy, often extending over a decade. CDMOs play a vital role in streamlining this process by offering expertise in formulating drugs, conducting necessary trials, and ensuring regulatory compliance. Their contribution is invaluable, particularly during the development and manufacturing organization stages where expertise and experience are crucial.
Commercial Manufacturing
CDMOs are integral to commercial manufacturing, providing a range of services that ensure products are manufactured efficiently and in compliance with regulatory standards. They cater to the needs of pharmaceutical companies by offering manufacturing services, analytical services, and continuous regulatory support.
Addressing Pharmaceutical Companies’ Pain Points
Regulatory Requirements
Navigating the complex landscape of regulatory requirements can be daunting for many pharmaceutical companies. CDMOs simplify this aspect by offering robust regulatory support services that guide companies from the initial submission strategy to post-approval requirements.
Supply Chain Management
Effective supply chain management is critical in the pharmaceutical industry to ensure timely delivery and maintain product quality. CDMOs provide comprehensive supply chain and logistics management services, optimizing distribution channels to meet market demands efficiently.
Cost-Effective Production
Reducing costs while maintaining high-quality production standards is a significant challenge in the industry. CDMOs help by offering cost-effective production solutions, leveraging their state-of-the-art facilities and expertise to deliver high-quality products at competitive costs.
Why Partner With a CDMO?
Technical Expertise
CDMOs bring unmatched technical expertise to the table, from drug discovery through to commercial production. Partnering with a CDMO means accessing years of accumulated knowledge and cutting-edge technology to develop and manufacture high-quality pharmaceutical products.
Flexible Manufacturing Capabilities
CDMOs offer flexible manufacturing capabilities that can be adjusted to meet the unique needs of different projects, whether for niche market drugs or high-volume commercial products.
Integrated Services for End-to-End Solutions
By offering an integrated service model, CDMOs can provide end-to-end solutions encompassing all phases of drug development and manufacturing. This approach ensures a seamless transition from one stage to the next, enhancing efficiency and productivity.
Key Components of Successful CDMO Partnerships
Clear Communication
Effective communication between the pharmaceutical company and the CDMO is critical for a successful partnership. Clear goals, timelines, and expectations should be established from the outset.
Comprehensive Planning
Detailed planning is crucial to navigate the complexities of drug development and manufacturing. Partnering CDMOs bring their expertise to develop thorough project plans that cover all stages of development and manufacturing.
Emphasis on Quality
Maintaining the highest quality standards is non-negotiable in the pharmaceutical industry. Reputable CDMOs adhere to stringent quality assurance and control measures to ensure compliance and product integrity.
Understanding and leveraging the extensive services offered by CDMOs can significantly enhance the drug development and manufacturing process for pharmaceutical companies. These organizations provide the expertise, facilities, and comprehensive support required to navigate the complexities of the pharmaceutical industry efficiently. By partnering with a reputable CDMO, companies can focus on their core competencies, reduce costs, and accelerate their journey from drug development to commercial success.
FAQs about CDMO Services
What is the difference between CRO and CDMO?
CROs (Contract Research Organizations)focus primarily on research and clinical trial services, while CDMOs (Contract Development and Manufacturing Organizations) provide comprehensive development and manufacturing services.
What is full-service CDMO?
A full-service CDMO offers end-to-end solutions for drug development and manufacturing, covering all stages from initial drug formulation to large-scale commercial manufacturing.
How do CDMO services improve the drug development timeline?
CDMO services streamline the drug development timeline by integrating various processes under one roof. This integration reduces hand-off delays, ensures greater coordination, and utilizes advanced technology and expertise to expedite each stage of development and manufacturing.
Why should pharmaceutical companies outsource to a CDMO?
Outsourcing to a CDMO allows pharmaceutical companies to leverage specialized expertise, state-of-the-art facilities, and comprehensive regulatory support. This collaboration can result in reduced costs, faster time-to-market, and the ability to focus on core competencies, such as drug discovery and innovation.
