Table of Contents
The pharmaceutical industry is a constantly changing and highly regulated world, hence understanding what CDMO pharma means is crucial to the success of pharmaceutical companies. Contract Development and Manufacturing Organizations (CDMOs) are the major players in this context since they provide all the services that are fully integrated and thus improve the whole development process of the drugs. This article will explain in greater detail what is a CDMO, its functions, advantages, as well as basic principles on how to choose the right CDMO partner.
What is a CDMO? Explained

Unlike other contract service providers in the pharmaceutical domain, CDMOs uniquely combine drug development expertise with manufacturing capabilities under one contract. A CDMO supports multifaceted activities including active pharmaceutical ingredient (API) development, formulation development, regulatory compliance, clinical trial management, process development, upscaling, and commercial production. By harnessing the power of advanced technologies, including AI machine learning, and comprehensive development services, CDMOs assist in streamlining processes, which allow biotech and pharma companies to not only cut costs but also improve their market entry timelines, while adhering to stringent regulatory requirements. The importance of CDMOs in pharma is further highlighted by their significant participation in both the creation and production of pharmaceutical products, making them critical partners in the pharmaceutical value chain.
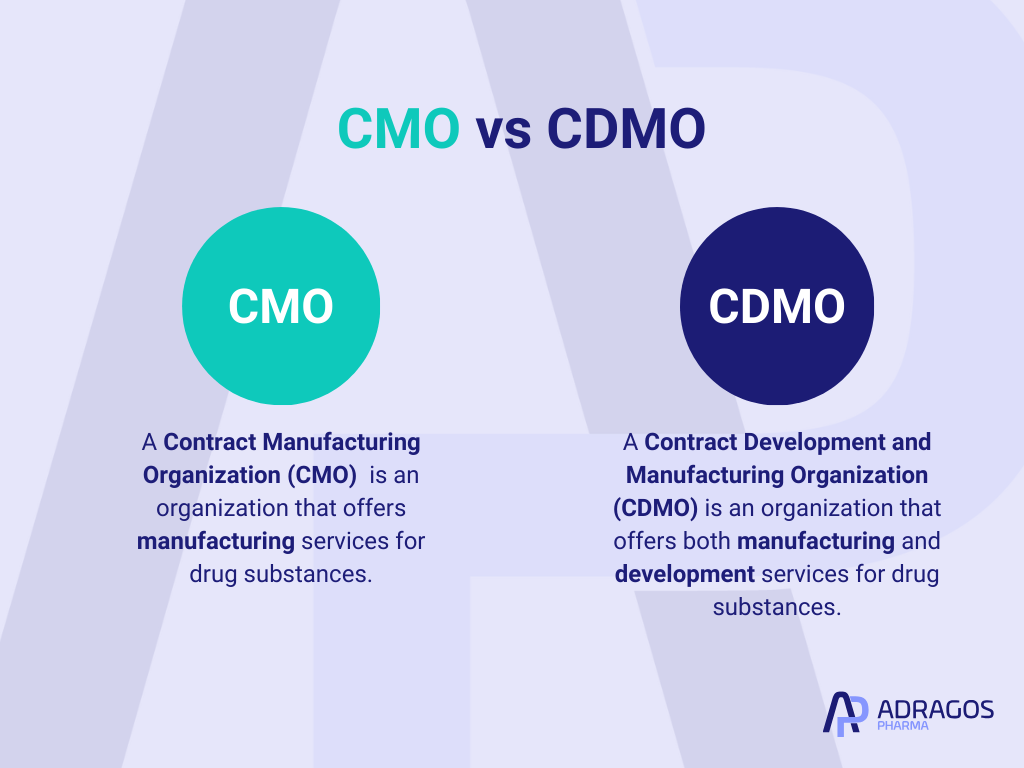
What is the difference between a CMO and CDMO?
A Contract Manufacturing Organization (CMO) and a Contract Development and Manufacturing Organization (CDMO) both play vital roles in the pharmaceutical and biotechnology industries, yet they serve distinctly different functions. A CMO is primarily focused on manufacturing, providing essential manufacturing services for companies looking to outsource the manufacturing process of their drugs, medical devices, or other health-related products. In contrast, a CDMO offers a more comprehensive range of services that extend beyond manufacturing to include product development. This includes tasks such as formulation development, process development, and sometimes even regulatory compliance—offering an all-in-one one-stop shop solution from concept through to commercial production.
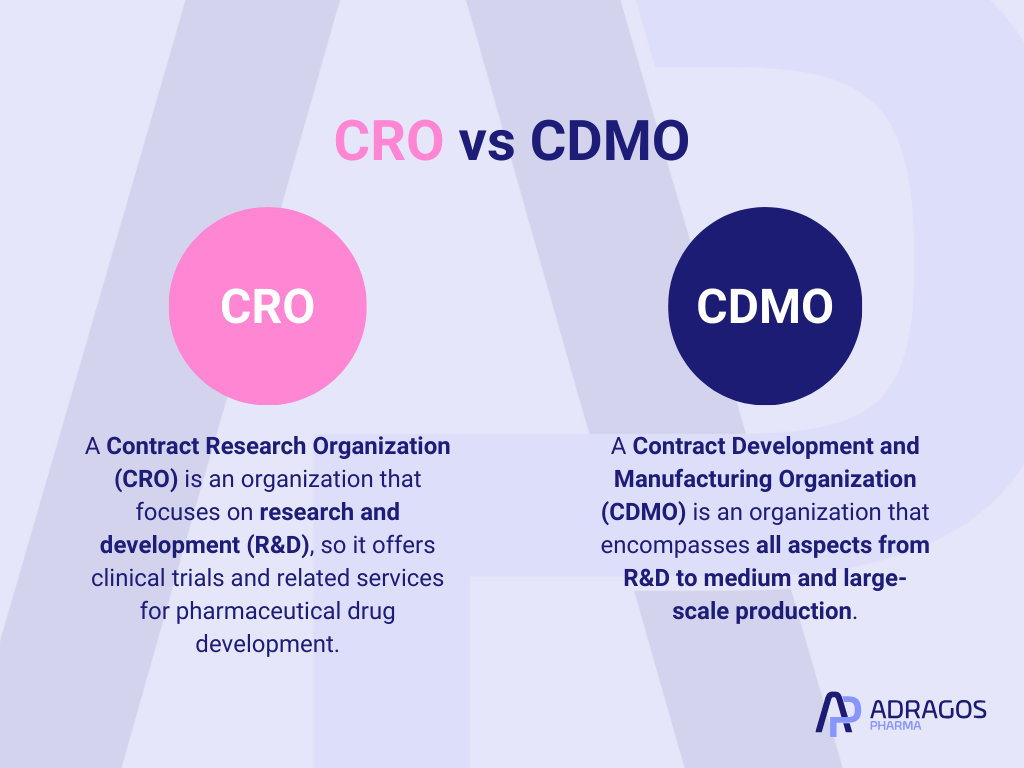
What is the difference between CRO and CDMO?
Understanding the differences between a Contract Research Organization (CRO) and a Contract Development and Manufacturing Organization (CDMO) is key to grasping their unique impacts on the drug development process. A CRO specializes in the research phase, delivering expertise in clinical trials, data management, and regulatory compliance to aid in bringing new drugs to the market. They are pivotal in handling the scientific and regulatory requirements necessary for drug approval. On the other hand, a CDMO bridges the gap between drug discovery and mass production, offering services from early stage development all the way to full service commercial scale drug manufacturing. This integration enables a smoother transition from laboratory breakthroughs to market-ready pharmaceutical products, positioning CDMOs as essential partners in scaling up production post-trial and approval phases.
Why use a CDMO?
The pharmaceutical industry encounters several challenges ranging from complex drug development processes, high operation costs, to stringent regulatory requirements. Here’s how a CDMO helps address these challenges in the pharmaceutical sector:
- Cost Effective Solutions: Pharmaceutical companies can significantly reduce or avoid the huge investment required for infrastructural development including labs and manufacturing facilities, as CDMOs make these facilities ready-for-use.
- Expertise Access: Leveraging the deep expertise and experience of CDMOs allows pharmaceutical companies to navigate the complexities of formulating drugs and regulating processes effectively, ensuring high standards of quality control and regulatory compliance.
- Flexibility and Speed: CDMOs facilitate greater agility in responding to market needs. They provide scalable solutions that help in managing fluctuating demand and can rapidly adjust production volumes to meet market and clinical trial services needs efficiently.
How to Choose the Right CDMO Partner
The choice of a CDMO partner can significantly impact the success of a pharmaceutical project. Here are some essential considerations when selecting a CDMO:
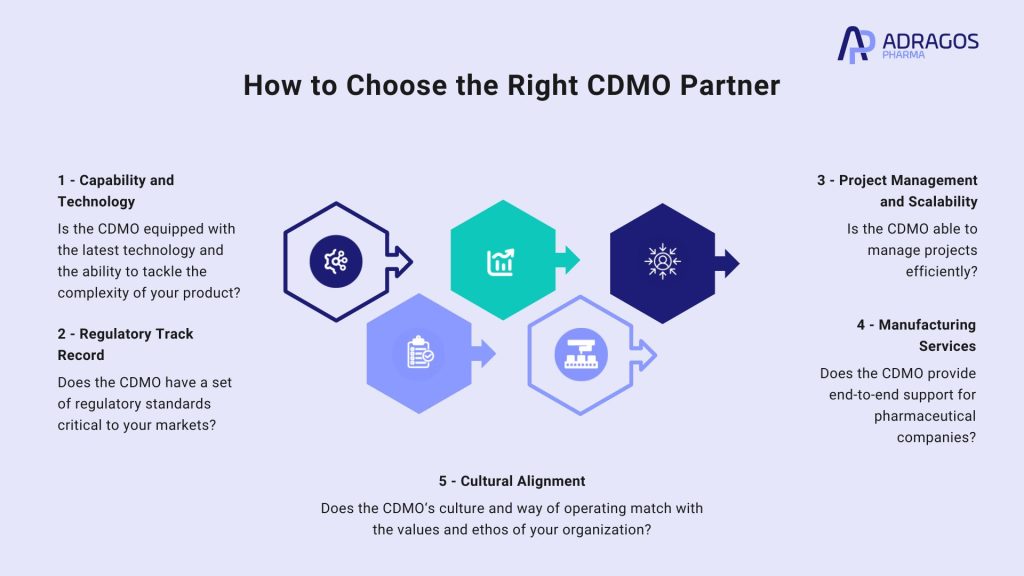
- Capability and Technology: Analyze if the CDMO is equipped with the latest technology and the ability to tackle the complexity of your product, especially if the product has unique features like nanoformulations, gene therapy, small molecule formulations, or antibody drug conjugates. Large-scale manufacturing facilities provided by CDMOs offer flexibility, collaboration, and innovation services that can accelerate drug development and lower costs.
- Regulatory Track Record: Ensure the CDMO has a well-established background of meeting regulatory requirements critical to your markets. This includes their adherence to cGMP – Current Good Manufacturing Practices.
- Project Management and Scalability: The ability of a CDMO to manage projects efficiently, while scaling processes to meet manufacturing demands without compromising on quality control, is critical.
- Manufacturing Services: CDMOs provide end-to-end support for pharmaceutical companies, including both research and manufacturing processes. This comprehensive approach makes them the preferred choice for companies seeking flexibility, consistency, and expertise in the manufacturing process.
- Cultural Alignment: The partnership can have a big impact on the time frame and the success of the product, therefore, it is very important that the CDMO’s culture and way of operating matches with the values and ethos of your organization.
Want to know more about how to select the right CDMO partner?
Challenges CDMOs Help Pharmaceutical Companies Overcome
In the rapidly evolving pharmaceutical industry, companies face numerous challenges that can hinder their progress from drug discovery to market delivery. Contract Development and Manufacturing Organizations (CDMOs) are pivotal in providing specialized solutions to these obstacles. By partnering with CDMOs, pharmaceutical companies can leverage external expertise and advanced technologies to enhance efficiency, comply with regulatory requirements, and streamline manufacturing processes. Below, we explore the key challenges in the pharmaceutical sector and the strategic support CDMOs offer to address them effectively:
Need for Advanced Manufacturing Technologies
CDMOs bring specialized expertise in complex drug formulations and biologics production, offering access to cutting-edge technologies such as spray drying and AI machine learning without the need for pharmaceutical companies to invest in expensive infrastructure.
Supply Chain Optimization
CDMOs streamline the supply chain by integrating development services and production processes, reducing inefficiencies, and ensuring a reliable flow of pharmaceutical products.
Scalability and Flexibility
CDMOs provide adaptable production capacities, which are essential during scale-up phases and when adjusting to market demands, ensuring efficient transitions from clinical trials to commercial production.
Cost Efficiency
By outsourcing production, pharmaceutical companies can significantly reduce capital and operational expenditures, as CDMOs manage the entirety of the manufacturing process, from raw material procurement to finished product.
Focus on Core Competencies
Outsourcing manufacturing processes to CDMOs allows pharmaceutical companies to concentrate on core competencies like R&D and marketing, enhancing innovation and market positioning.
Speed to Market
CDMOs streamline the manufacturing and quality control processes, ensuring that products not only meet regulatory compliance standards quickly but also reach the market faster, thus providing a competitive edge.
Risk Mitigation
Solution: CDMOs mitigate production and supply chain risks by employing their deep expertise and robust quality control systems, ensuring consistent product development and supply reliability.
Regulatory Compliance
Solution: CDMOs handle the complex regulatory requirements across different markets, ensuring compliance and minimizing risks, which simplifies the approval process for new drugs and accelerates market entry.
Benefits of Engaging with a CDMO
Collaborating with a CDMO provides numerous benefits to pharmaceutical companies aiming to streamline their operations and market strategies:
- Enhanced Efficiency: By consolidating research and development services with manufacturing processes, CDMOs can reduce complexities involved in the development process, ensuring smoother transitions from early-stage development to commercial production.
- Reduced Overheads: Partnerships with CDMOs can significantly lower capital and operational expenditures by eliminating the need to invest heavily in infrastructure and manufacturing facilities.
- Market Responsiveness: Utilizing the integrated services and expertise of CDMOs allows pharmaceutical companies to quickly adapt to changes in market demand or regulatory compliance landscapes, thereby maintaining competitive advantages.
CDMOs have become indispensable in the pharmaceutical industry by allowing companies to focus more on their core competencies like drug discovery and market strategy while outsourcing the complex, resource-intensive tasks of pharmaceutical development and manufacturing. As pharmaceutical companies increasingly seek efficiencies in their product pipelines, the strategic importance of CDMOs is growing day by day, underpinning their critical role in the delivery of safe, effective, and innovative drugs to the market.growing day by day, underpinning their critical role in the delivery of safe, effective, and innovative drugs to the market.

FAQs about CDMO
1. How does CDMOs work?
CDMOs provide an integrated approach by combining drug development services with manufacturing capabilities for pharmaceutical and biotechnology companies. This allows them to manage and streamline the entire process from initial research, through development stages, up to full-scale commercial production.
2. Why do companies use CDMO?
Pharmaceutical companies engage CDMOs to leverage their integrated services, which help pharma companies reduce operational costs and developmental risks while enhancing efficiency and speed in bringing producost effectivects to the market.
3. How can partnering with a CDMO accelerate the drug development process for pharmaceutical companies?
Partnering with a CDMO can significantly accelerate the product development timeline for pharmaceutical companies by integrating various stages of the drug development process. CDMOs offer expertise in formulation, regulatory compliance, and scale-up activities, which enables them to efficiently manage and execute complex projects from conceptualization to market readiness, thus shortening the cycle time necessary to launch new products.
4. What strategic advantages do CDMOs offer to pharmaceutical businesses looking to expand into new markets through commercial manufacturing?
CDMOs provide pharmaceutical businesses with strategic advantages such as access to state-of-the-art technologies, regulatory expertise for global markets, and adaptable manufacturing capabilities. This support is crucial for companies aiming to enter new therapeutic areas or geographical markets, as it allows them to navigate unfamiliar regulatory environments and scale production without significant upfront investments in facilities or technology.
5. How do CDMOs ensure compliance with international regulatory standards?
CDMOs ensure compliance with international regulatory standards by maintaining up-to-date certifications and adhering to global best practices, such as following Current Good Manufacturing Practices (cGMP). They regularly undergo audits and inspections to ensure their processes and facilities meet all necessary regulatory requirements. Besides, a lot of CDMOs have regulatory affairs departments that take care of the changes in regulatory landscapes across different regions.
6. Can CDMOs provide tailored solutions to fit the specific needs of different pharmaceutical companies?
Yes, CDMOs are well-equipped to provide tailored solutions that meet the specific needs of different pharmaceutical companies. This includes custom development services, proprietary process optimizations, and specialized manufacturing setups that cater to unique product requirements, small batch production for niche markets, or large-scale production for widespread distribution. The flexibility and customer-centric approach of CDMOs make them valuable partners in addressing diverse and specialized demands.
7. What is the meaning of CDMO?
A CDMO stands for Contract Development and Manufacturing Organization. CDMOs provide both drug development and manufacturing services to pharmaceutical and biotechnology companies.
8. What is the difference between a CMO and a CDMO?
A CMO (Contract Manufacturing Organization) offers manufacturing services only, while a CDMO provides both drug development and manufacturing services, making CDMOs a one-stop solution for both development and production needs.
