Table of Contents
In the pharmaceutical sciences, drug formulation is a cornerstone of transforming a promising compound into a safe, effective, and patient-friendly therapeutic product. In this comprehensive overview, we explore the critical aspects of drug formulation, its challenges, and its significance in the broader landscape of drug development.
What is Drug Formulation
Drug formulation (or pharmaceutical formulation) refers to the process of combining various pharmaceutical ingredients, both active and inactive, to produce a final drug product.
Drug formulation consits in combining various inactive substances, also known as excipients, with the active pharmaceutical ingredient (API), also known as the drug substance, to produce a final product for patients.
The primary goal is to create a dosage form that ensures the drug’s bioavailability, stability, and patient compliance. The compatibility between the inactive substance and the drug substance is crucial, as excipients play a significant role in drug release and therapeutic effectiveness. Ensuring that the excipients and API work harmoniously together can greatly enhance the overall performance and safety of the pharmaceutical product.
The Role of Drug Formulation in Pharmaceutical Development
The importance of drug formulation cannot be overstated. A well-crafted formulation can significantly reduce adverse effects, improve patient compliance, and enhance the drug’s shelf life. It is especially vital during clinical trials, where consistent and reliable formulations are necessary to accurately assess a drug’s therapeutic effects. Moreover, successful pharmaceutical formulations are essential for ensuring that a drug product can be mass-produced and distributed effectively.
Types of Drug Formulations
In the realm of pharmaceutical formulation, there are three primary types of formulations: solid formulations, liquid formulations, and parenteral formulations. Each type serves a unique purpose and is selected based on the drug’s chemical properties, intended route of administration, and patient compliance considerations.
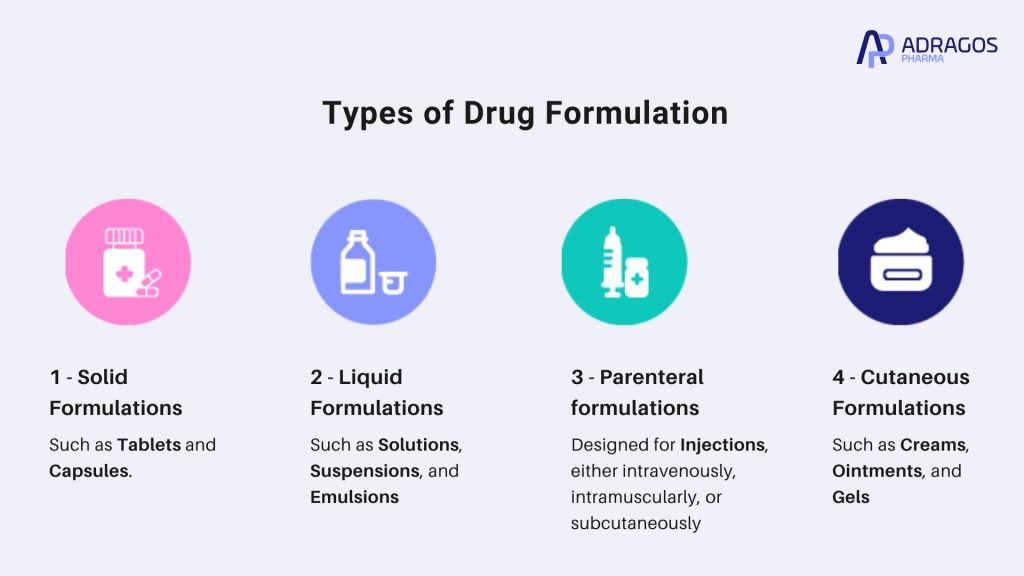
Solid Formulations
Solid formulations are among the most common and include dosage forms like tablets and capsules. Tablets typically consist of a compressed mixture of the active pharmaceutical ingredient (API) and inactive ingredients, which help in the tablet’s stability and dissolution in the digestive system. Capsules, on the other hand, enclose the active ingredient in a gelatin shell that dissolves in the stomach, making it easier for patients to ingest.
Liquid Formulations
Liquid formulations encompass solutions, suspensions, and emulsions. These are often chosen for their ease of administration, especially in pediatric patients or those who have difficulty swallowing tablets. They offer rapid absorption and can be adjusted in concentration to suit specific dosage requirements.
Parenteral formulations
Parenteral formulations are designed for injection, either intravenously, intramuscularly, or subcutaneously. These formulations bypass the gastrointestinal tract, providing immediate and complete delivery of the drug into the bloodstream. They are crucial for medications that are not effective or stable when taken orally, such as biologics and other complex drug products.
Cutaneous Formulations
Topical formulations, such as creams, ointments, and gels, are applied directly to the skin. They are ideal for treating localized conditions and ensuring that the drug acts directly at the site of application.
Pharmaceutical Formulation Techniques
Granulation and Its Importance
Granulation is one of the foundational techniques in the preparation of solid dosage forms, especially tablets and capsules. This process involves forming powders into larger, multi-particle entities called granules. Wet granulation uses a granulating fluid, while dry granulation relies on compaction. This technique improves flowability and compressibility of powders, ensures the even distribution of the active pharmaceutical ingredient (API), and prevents segregation of ingredients. All these factors are critical for achieving accurate dosing and product stability.
Encapsulation and Controlled Release
Encapsulation is a versatile technique that allows manufacturers to encase APIs in a shell, typically gelatin or a plant-based alternative, resulting in capsules. This not only facilitates precise dosing, but also helps mask unpleasant tastes or odors associated with certain drugs. Encapsulation technology has evolved to include various types such as soft gels, hard gels, and even microcapsules, each serving specific therapeutic needs. Furthermore, controlled-release and extended-release formulations are achievable with modern encapsulation, allowing for drugs to be released at predetermined rates and intervals, thus improving treatment efficacy and patient adherence.
Advanced Techniques: Lyophilization, Nanotechnology, and Microencapsulation
Modern pharmaceutical formulation employs advanced technologies to address challenges with certain drug compounds. Lyophilization (or freeze-drying) is commonly used for biologics and vaccines; it removes water under low temperatures, preserving the activity and stability of sensitive molecules. Nanotechnology—especially nanoparticle engineering—enables drugs with poor water solubility to have increased bioavailability and therapeutic potential. Microencapsulation encloses drugs in microscopic carriers, enabling targeted delivery, reduced side effects, and sustained drug release. The use of polymers and biodegradable materials in microencapsulation further supports innovative drug delivery solutions tailored to specific diseases and patient requirements.
Benefits of Pharmaceutical Formulation
Enhanced Drug Efficacy and Consistency
A primary benefit of pharmaceutical formulation is the improvement of drug efficacy and consistency. Through engineered delivery mechanisms and optimized release profiles, drugs can exert their intended therapeutic effects more reliably. Proper formulation ensures that each dose contains a uniform amount of the API, reducing the risk of under- or overdosing—a critical factor for patient safety and treatment effectiveness.
Improved Stability and Shelf Life
Pharmaceutical formulations play a vital role in stabilizing sensitive compounds and extending shelf life. Drugs are protected from environmental factors such as light, humidity, oxygen, and temperature fluctuations through the incorporation of stabilizing agents or specialized packaging. These measures prevent degradation and loss of potency, reducing product wastage for both manufacturers and patients. Common strategies include using antioxidants, preservatives, and desiccants to safeguard the integrity of pharmaceutical products throughout their lifespan.
Better Patient Compliance and Targeted Therapy
Well-designed formulations cater to diverse patient needs, making it easier for patients to comply with their treatment regimens. Innovations like chewable tablets, flavored liquids, orally disintegrating tablets, and transdermal patches provide alternative administration routes for children, the elderly, and those with swallowing difficulties. Moreover, advanced formulation technologies enable targeted drug delivery, ensuring the drug reaches specific tissues or organs. This maximizes therapeutic outcomes while minimizing systemic side effects, increasing the success rate of treatments and enhancing patients’ quality of life.
Economic and Operational Advantages
Formulation development also offers operational and economic gains. Enhanced stability leads to longer shelf lives, reducing the frequency of drug recalls and contributing to cost savings for both manufacturers and healthcare providers. A robust, scalable formulation process ensures the reliable supply and accessibility of essential medications without compromising quality.
Challenges in Drug Formulation Development
Solubility and Bioavailability
A significant challenge in drug formulation development is addressing poor solubility and low bioavailability of many active pharmaceutical ingredients. Pharmaceutical scientists often need to employ various techniques to enhance these properties, ensuring that the drug can be effectively absorbed by the body.
Stability and Shelf Life
Ensuring the drug product’s stability throughout its shelf life is critical. This involves preventing degradation or polymorphic changes that could affect the drug’s efficacy. Techniques such as stabilizing agents or protective packaging are often used to address these issues.
Dose Range and Dosage Forms
Determining the correct dose range and appropriate dosage forms is another complex aspect of drug formulation. This process involves balancing the drug’s therapeutic effect with safety and minimizing side effects.
Manufacturing Scalability
Another challenge is ensuring that the drug formulation can be scaled up from laboratory to industrial production without compromising quality. This requires robust process development and optimization to ensure consistency in large-scale manufacturing.
Regulatory Compliance
Compliance with regulatory standards, such as those set by the FDA, EMA, or other authorities, is crucial. This involves extensive testing, documentation, and adherence to Good Manufacturing Practices (GMP).
Environmental and Sustainability Concerns
There is increasing pressure to develop formulations and manufacturing processes that are environmentally friendly. This includes minimizing waste, using sustainable raw materials, and reducing energy consumption.
Recent Advances in Drug Formulation
The field of formulation development has seen significant advancements, particularly with the integration of technologies like deep learning and advanced analytical tools. These innovations have enabled the creation of novel drug formulations that cater to specific needs, such as complex generics and various dosage forms.
In conclusion, drug formulation is a critical aspect of the drug development process, playing a pivotal role in ensuring that pharmaceutical formulations are safe, effective, and patient-friendly. From the initial concept to the final product, the meticulous work involved in formulation development ensures that drugs meet the highest standards of quality and efficacy.
FAQs about Drug Formulation
What are the three types of formulations?
The three primary types of formulations are solid formulations (e.g., tablets, capsules), liquid formulations (e.g., solutions, suspensions), and parenteral formulations (e.g., injections).
What are the examples of drug formulation?
Examples of drug formulation include tablets, capsules, creams, ointments, syrups, injectables, and inhalers. Each formulation is chosen based on the drug’s properties, therapeutic goals, and patient requirements.
What are different steps of drug formulation?
The typical steps in drug formulation include preformulation studies (evaluating the drug’s properties), selection of excipients, developing the drug-excipient mixture, preparing the dosage form (such as tablet compression or encapsulation), conducting stability testing, and scaling up for manufacturing.
How many main types of medication formulations are there?
There are six main types of medication formulations: tablets, capsules, liquids, sustained release, parenteral, and topical formulations.
What is meant by drug formulation?
Drug formulation refers to the process of designing and preparing a drug product by combining the active pharmaceutical ingredient (API) with suitable inactive components (excipients) to create a stable, effective, and safe dosage form for patients.
What is the goal of drug formulation?
The primary goal of drug formulation is to deliver the active pharmaceutical ingredient in a manner that ensures optimal stability, bioavailability, safety, and patient acceptability, thereby maximizing therapeutic outcomes.
