In the complex and ever-evolving pharmaceutical industry, understanding the distinctions between drug substance and drug product is crucial for pharmaceutical companies engaged in drug development. These terms, while often used interchangeably, refer to very different components within the lifecycle of a medication. In this comprehensive guide, we’ll explore these differences, starting with their definitions and diving into their roles in the manufacturing and regulatory processes. Whether you’re new to the field or an experienced professional, this article will clarify these critical concepts.
What is a Drug Substance?
A drug substance, also known as the active pharmaceutical ingredient (API), is the core component of any medication. It is the pure material that has a pharmacological action or direct effect on the body, contributing to the drug’s pharmacological activity and intended therapeutic effect. The drug substance is responsible for the drug’s pharmacological activity, making it the most critical component in the manufacturing process.
The drug substance can be derived from various sources, including natural sources, chemical synthesis, or biotechnology. Its purity standards are strictly monitored by regulatory authorities like the Food and Drug Administration (FDA) to ensure patient safety and efficacy. The drug substance is often subject to quality control tests to confirm that it meets the required acceptance criteria before it can be used in further drug product development.
What is a Drug Product?
As the Food and Drug Administration (FDA) states, a Drug Product can be defined as the finished dosage form that contains a drug substance, generally, but not necessarily in association with other active or inactive ingredients. Unlike intermediate products, a drug product is ready for market use and does not require further processing.
The drug product can come in various forms, including tablets, capsules, injectables, and more. It is a complex mixture that not only contains the drug substance but also other components like excipients, packaging, and labeling that are crucial for the drug’s efficacy, stability, and safety. The FDA and other regulatory authorities oversee the manufacturing processes to ensure that each drug product meets strict quality and safety standards.
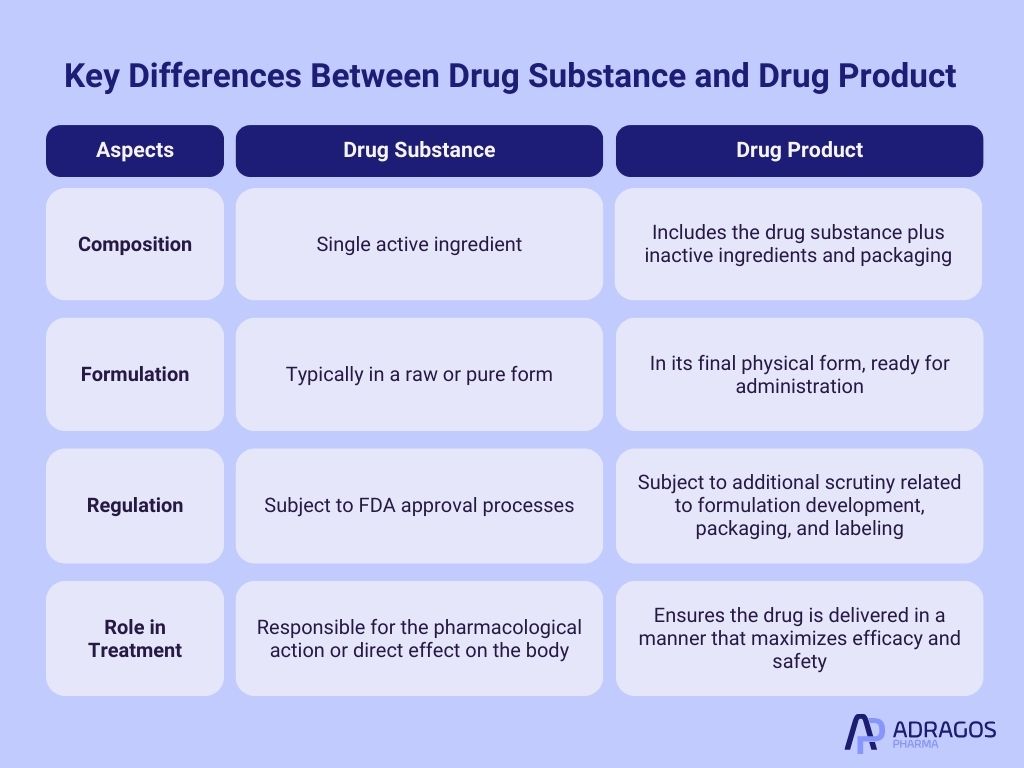
Drug Product vs Drug Substance: Key Differences
While the drug substance is the active element that causes the desired therapeutic effect, the drug product is the complete package that is administered to the patient. Here’s a breakdown of the key differences:
Composition: The drug substance is a single active ingredient, while the drug product includes the drug substance plus inactive ingredients and packaging.
Formulation: The drug substance is typically in a raw or pure form, whereas the drug product is in its final physical form ready for administration.
Regulation: Both the drug substance and drug product are subject to stringent FDA approval processes, but the drug product undergoes additional scrutiny related to its formulation development, packaging, and labeling.
Role in Treatment: The drug substance is responsible for the pharmacological action or direct effect on the body, while the drug product ensures that the drug is delivered in a manner that maximizes its efficacy and safety.
The Importance of Drug Product Development
Drug product development is a critical phase in bringing a new medication to the market. This phase often involves collaboration with Contract Development and Manufacturing Organizations (CDMOs) that provide comprehensive pharmaceutical manufacturing services. It involves converting the drug substance into a usable finished product that meets all regulatory requirements and is safe for patient use. This process includes the selection of appropriate excipients, designing the dosage form, and ensuring the stability and bioavailability of the drug product.
During this phase, pharmaceutical companies must focus on various aspects of formulation development, including the physical and chemical properties of the drug substance, the compatibility of excipients, and the optimization of the manufacturing process. The goal is to create a drug product that is not only effective but also convenient for the patient to use, whether it’s a tablet, capsule, or injectable.
Regulatory Affairs and Quality Control
The FDA and other regulatory authorities play a crucial role in ensuring the safety and efficacy of both drug substances and drug products. The approval process for a new drug is rigorous, involving multiple stages of testing and review. Quality control measures are in place throughout the manufacturing and development processes to ensure that every batch of drug product meets the required standards.
Pharmaceutical companies must navigate complex regulatory affairs to bring a new drug to market. This includes submitting drug applications, conducting clinical trials, and maintaining compliance with FDA regulations. The focus on patient safety and efficacy is paramount, and any deviation from quality standards can result in significant delays or even rejection of the drug product.
Understanding the distinction between a drug substance and a drug product is essential for anyone involved in the pharmaceutical industry. The drug substance is the active ingredient that drives the therapeutic effect, while the drug product is the complete, ready-to-use medication. Both elements are critical to the success of a medication, and each plays a unique role in ensuring that the final product is safe, effective, and accessible to patients.
FAQs about drug product vs drug substance
What is the difference between a drug product and a substance?
A drug substance is the pure active ingredient responsible for the therapeutic effect of a drug. In contrast, a drug product includes the drug substance along with inactive ingredients, packaging, and other elements that make the drug ready for patient use.
What is the difference between DS and DP?
DS (Drug Substance) refers to the active ingredient with pharmacological activity, while DP (Drug Product) is the final form of the medication that includes the DS, excipients, and packaging.
What is considered a drug product?
A drug product is the finished dosage form of a drug that is ready for administration to patients. It includes the drug substance along with any inactive ingredients and packaging.
What is the definition of a drug substance?
A drug substance is the active ingredient in a medication that has a pharmacological action and contributes to the intended therapeutic effect. It is also known as the active pharmaceutical ingredient (API).
