In the rapidly evolving field of pharmaceuticals, modified release tablets stand out as a significant advancement that optimizes drug delivery for better patient outcomes. This article delves into the intricacies of modified release technologies, offering a comprehensive view tailored to the needs and interests of pharmaceutical professionals, from newcomers to seasoned experts.
What are Modified Release Tablets?
Modified release tablets, also known as modified release dosage forms, are engineered to release their active ingredients at a predetermined rate, location, and time, offering an improvement over conventional immediate release formulations that release their contents quickly and completely after ingestion. This technology encompasses several types of release mechanisms, such as extended release (ER), sustained release (SR), controlled release (CR), and delayed release (DR), each tailored to address specific therapeutic needs and improve patient compliance.
Benefits of Modified Release Tablets
Modified release tablets offer several significant benefits over traditional immediate release formulations. One of the primary advantages is improved patient compliance, as these tablets often require less frequent dosing. This reduction in dosing frequency can lead to better adherence to treatment regimens, ultimately resulting in improved therapeutic outcomes. Additionally, modified release tablets provide a more consistent and sustained release of the active ingredient, which helps in reducing peak-to-trough fluctuations in serum drug concentration. This stability can lead to a reduction in side effects and enhanced efficacy.
Moreover, modified release tablets can be engineered to release the active ingredient at a specific time or over a prolonged period, allowing for more targeted and efficient drug delivery. This precision in drug delivery ensures that the medication is released when and where it is needed most, optimizing the therapeutic effect and minimizing potential side effects. By offering a controlled and sustained release, these tablets enhance the overall management of chronic conditions, making them a valuable tool in modern clinical pharmacology.
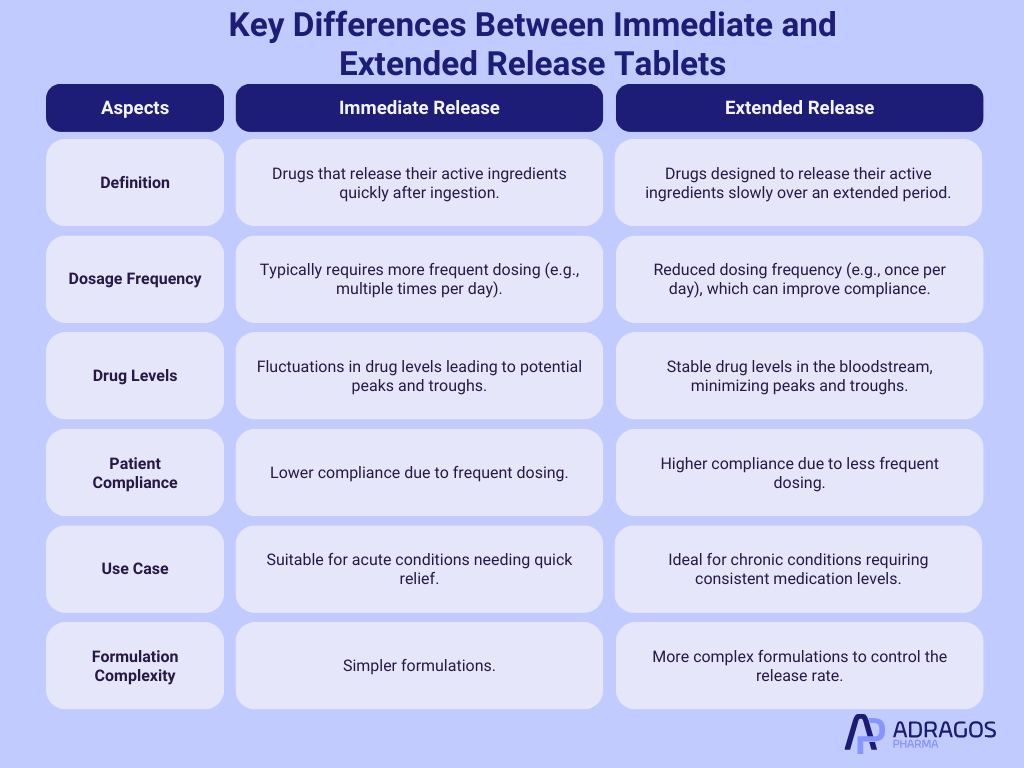
Immediate vs. Extended Release: Understanding the Difference
It’s crucial to distinguish between immediate-release (IR) and extended-release (ER) drugs by understanding their release dosage. Immediate-release drugs are designed to dissolve quickly, delivering the medication into the bloodstream shortly after administration. In contrast, extended-release drugs are formulated to release their active components more slowly over an extended period. This not only maintains the therapeutic effect longer but also minimizes the frequency of dosing—a major advantage in managing chronic conditions.
Types of Modified Release Systems
There are several types of modified release systems, each designed to meet specific therapeutic needs and improve drug delivery. Sustained release systems are formulated to release the active ingredient over a prolonged period, maintaining a constant drug concentration in the bloodstream. This steady release helps in managing conditions that require consistent medication levels, such as chronic pain or hypertension.
Controlled release systems, on the other hand, release the active ingredient at a predetermined rate, allowing for more precise control over drug delivery. This type of system is particularly beneficial for medications that need to maintain a specific therapeutic window. Delayed release systems are designed to release the active ingredient at a specific time, often after a delay period. This can be useful for drugs that need to bypass the acidic environment of the stomach and be released in the intestines.
Other innovative types of modified release systems include programmed drug delivery systems, which release the active ingredient in a predetermined pattern, and targeted drug delivery systems, which release the active ingredient at a specific site in the body. These advanced systems offer tailored solutions for complex therapeutic needs, enhancing the efficacy and safety of treatments.
The Role of Slow-Release Drugs
Slow-release drugs, including various forms of modified release tablets, are designed to serve specific purposes:
- Enhancing patient compliance by reducing the number of doses needed per day.
- Stabilizing drug levels in the blood, thereby avoiding peaks and troughs associated with multiple dosing.
- Improving treatment outcomes by maintaining consistent drug levels.
- Reducing side effects by avoiding high concentrations of the drug in the bloodstream.
Formulation and Design Considerations
The formulation and design of modified release tablets require meticulous consideration of several factors to ensure optimal performance. The properties of the active ingredient, such as its solubility, stability, and absorption characteristics, play a crucial role in determining the appropriate release profile. The choice of excipients, including binders, fillers, and lubricants, can significantly impact the release characteristics of the tablet.
The design of the tablet itself, including its shape, size, and coating, also influences the release profile. For instance, the use of specific coatings can protect the active ingredient from the acidic environment of the stomach, allowing for delayed release in the intestines. Additionally, the manufacturing process, including compression, granulation, and coating techniques, must be carefully controlled to ensure consistency and quality.
Thorough testing and evaluation are essential to confirm that the modified release tablet meets the desired specifications and performance characteristics. This includes in vitro dissolution testing and in vivo studies in healthy volunteers to assess the release profile and therapeutic effect. By addressing these formulation and design considerations, pharmaceutical companies can develop effective and reliable modified release medications.
Key Considerations in Prescribing Modified-Release Medications
When prescribing modified-release formulations, healthcare providers must consider several factors:
Patient-specific factors, such as age, body weight, kidney function, and the ability to swallow pills.
Monitoring blood pressure during prolonged use of certain medications is crucial to manage potential side effects.
Swallowing difficulties should be considered, and alternative methods of administration, like sprinkling contents on soft food, may be necessary.
Drug properties, such as its half-life, absorption site in the gastrointestinal tract, and interaction with food.
Disease characteristics that dictate the need for stable blood levels of drugs over prolonged periods.
These considerations ensure that each patient receives the most appropriate form of medication tailored to their specific needs.
Emerging Trends and Technologies
The field of modified release tablets is continually evolving, with several emerging trends and technologies shaping its future. One notable trend is the use of novel excipients, such as nanoparticles and liposomes, which can enhance drug delivery and improve the release profiles of active ingredients. These advanced excipients offer new possibilities for creating more effective and targeted modified release systems.
Innovative manufacturing techniques, such as 3D printing and hot melt extrusion, are also gaining traction. These technologies allow for greater precision and customization in the production of modified release tablets, enabling the development of complex dosage forms that were previously unattainable. Additionally, there is a growing interest in using modified release tablets for the delivery of biologics and other complex molecules, expanding the scope of this technology beyond traditional small-molecule drugs.
The use of modified release tablets for the treatment of chronic diseases, such as diabetes and cardiovascular disease, is another area of increasing interest. These tablets can provide more consistent and effective management of chronic conditions, improving patient outcomes. Furthermore, the development of personalized medicine and the use of modified release tablets for targeted drug delivery are emerging trends that hold great promise for the future of pharmaceutical care. By staying at the forefront of these advancements, pharmaceutical companies can continue to innovate and meet the evolving needs of modern healthcare.
Our Expertise in Tablet Manufacturing
At Adragos, particularly at our facility in Kawagoe, Japan, we specialize in the manufacturing of high-quality modified release tablets. Our capabilities range from small-scale batches for clinical trials to large commercial manufacturing, with advanced technologies in:
- Tablet compression
- Granulation, including semi-continuous techniques for optimal consistency and quality
- Film coating, utilizing both aqueous and solvent-based processes to ensure protection and controlled release of the active ingredients
Modified release tablets represent a significant advancement in pharmaceutical technology, providing enhanced control over drug delivery. This improves patient adherence, optimizes therapeutic effects, and minimizes side effects, marking a pivotal shift in chronic disease management and overall patient care.
Through innovations and expertise in drug formulation and manufacturing, pharmaceutical companies are poised to meet the complex demands of modern healthcare, ensuring that patients receive the most effective and manageable treatment options available.
FAQs About Modified Release Tablets
What is a Modified Release Drug?
A modified release drug is formulated to release its active ingredients over time in a controlled manner. This can be achieved through various technologies embedded within the tablet or capsule to prevent immediate drug release after ingestion.
What is the Difference Between Modified Release and Prolonged Release?
While both terms often refer to extended drug release intervals, “modified release” is an umbrella term that includes any alteration in the timing and/or place of release within the gastrointestinal tract, whereas “prolonged release” specifically extends the release duration compared to the conventional form.
What are Modified Release Opioids?
Modified release opioids are designed to provide prolonged pain relief by controlling the drug’s release rate. This formulation helps manage chronic pain more effectively by maintaining steady drug levels in the body.
What are Examples of Sustained-Release Tablets?
Examples of sustained-release tablets include medications like bupropion SR, which is used for depression management, and glipizide SR for diabetes treatment. These tablets slowly release medication to maintain a therapeutic effect over an extended period without repeated dosing.
