The pharmaceutical industry has witnessed significant advancements in generic drugs and their improved counterparts, super generics. These medicines play a crucial role in global healthcare, offering cost-effective alternatives to brand-name drugs while maintaining the same active ingredient and therapeutic equivalence.
With patent protection on many pharmaceutical products expiring, pharmaceutical companies are focusing on innovation in drug development to stay competitive. This has led to the rise of super generics, which provide patients with improved drug formulations that enhance efficacy, safety, and overall treatment outcomes.
In this article, we will explore the difference between generic drugs, generic medicines, and super generics, highlighting their market exclusivity, regulatory processes, and impact on the healthcare sector.
What Are Generic Drugs?
Generic drugs are pharmaceutical formulations that contain the same active ingredient as a brand-name drug. They offer the same therapeutic effect, safety, and efficacy but are typically sold at lower costs because they do not require extensive clinical trials like the original product.
Key Characteristics of Generic Drugs:
Contain the same active ingredients as the brand-name drug, ensuring therapeutic equivalence despite potential differences in inactive components
Undergo rigorous regulatory approval for safety and efficacy
Available at a significantly lower price due to the absence of patent protection costs
Widely used across many countries, making up over 91% of prescriptions in the U.S.
Understanding Generic Medicines and Their Impact on Healthcare
The availability of generic medicines significantly reduces pharmaceutical spending, benefiting both patients and healthcare systems. Retail pharmacies offer a variety of generic versions, helping patients access affordable treatments without compromising on quality.
Why Are Generic Medicines Important?
Cost Savings: Reduce the price paid by both healthcare providers and consumers
Increased Access: Enable wider distribution of essential medicines
Encouraging Competition: Promote generic competition and drive down drug costs
EU member states and the European Commission have emphasized the importance of generic medicines in improving public health while ensuring sustainable health economics.
How Generic Manufacturers Operate
Generic manufacturers play a crucial role in the pharmaceuticals supply chain by producing generic products that are bioequivalent to brand-name drugs. They ensure adherence to regulatory guidelines while maintaining high quality standards.
Key responsibilities of generic companies include:
Manufacturing and distributing generic versions of brand-name drugs
Conducting clinical trials to prove therapeutic equivalence
Meeting regulatory requirements set by the FDA and EMA
What Happens When Patent Protection Expires?
Once a patent expires, generic drugs can enter the market, increasing competition and reducing costs. This allows patients to access medicines at a lower price while still benefiting from the same active ingredient and therapeutic effect.
Impact of Patent Expiry on the Market
Increased competition among generic manufacturers
Decline in price of pharmaceutical products
Shift in sales from brand-name drugs to generic medicines
However, to maintain market exclusivity, pharmaceutical companies are turning to super generics, offering improved formulations with enhanced efficacy and safety.
What Are Super Generics?
Super generics, also known as complex generics or value-added generics, are modified versions of generic drugs that offer better therapeutic effect, enhanced drug delivery mechanisms, or improved safety data. These pharmaceutical products provide patients with more effective treatment options while ensuring market exclusivity for generic companies.
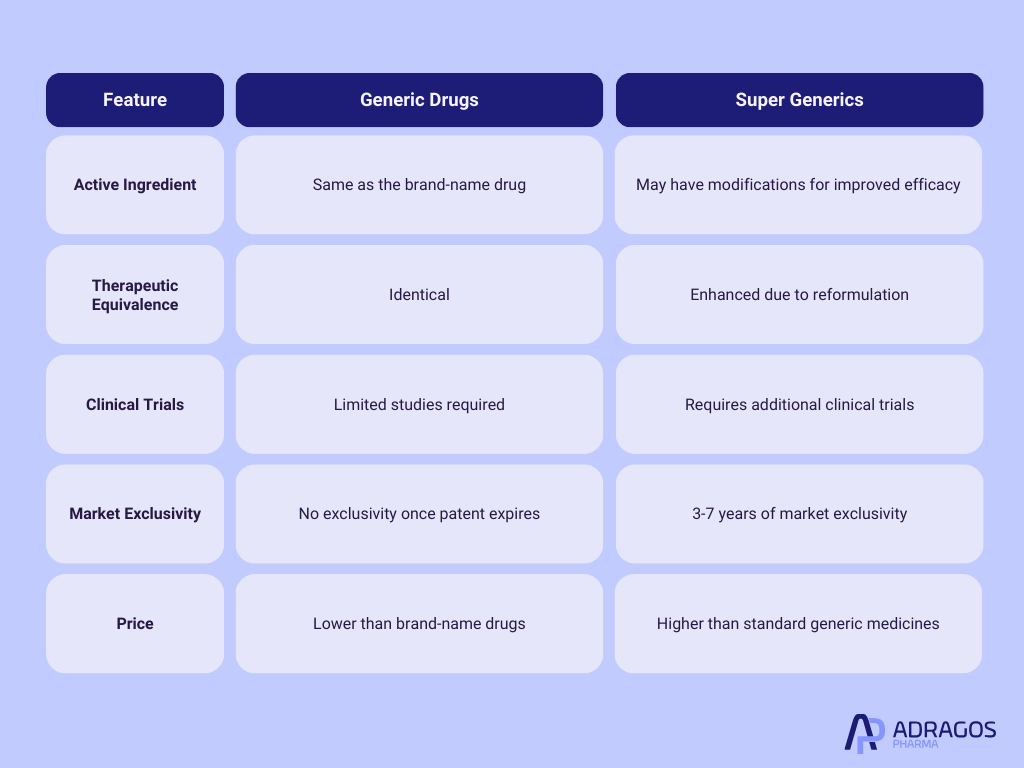
Key Differences Between Generic Drugs and Super Generics
The Role of Super Generics in Pharmaceutical Innovation
The development of super generics is a strategic move for pharmaceutical companies aiming to sustain profitability while addressing competition. Super generics offer:
Better absorption and efficacy
Lower risk of side effects
Extended-release formulations for improved patient compliance
With more than 32,000 generic products approved by the FDA in 2022 alone, the generics industry is becoming increasingly competitive. Pharmaceutical companies must continue to develop value-added solutions to differentiate their medicines from traditional generic drugs.
Regulatory Pathway for Super Generics
Unlike traditional generic medicines, super generics undergo rigorous clinical trials and regulatory reviews to prove their therapeutic equivalence.
In the United States, the FDA assesses the drug through the 505(b)(2) pathway, which allows modified formulations of existing pharmaceutical products to enter the market with new clinical evidence.
In Europe, the EMA follows a similar pathway, evaluating super generics under stringent guidelines to ensure their quality, safety, and efficacy before granting market exclusivity for a period of 3-7 years.
Both the FDA and EMA assess:
Safety data from preclinical studies
Efficacy improvements over standard generic drugs
Compliance with strict quality standards
Due to their innovation, super generics receive market exclusivity, allowing companies to generate higher sales and profits.
The Future of Generic and Super Generic Markets
The generic market continues to grow, fueled by patent expirations and rising demand for cost-effective medicines. However, increasing competition is pushing pharmaceutical companies to develop innovative formulations that offer unique benefits.
Key Market Trends:
Expansion of generic competition in EU member states
Growing demand for value-added generics in the healthcare sector
Increasing focus on regulatory approvals for super generics
With pharmaceutical spending on the rise, health economics will continue to shape the strategy of generic manufacturers worldwide.
The difference between generic drugs and super generics lies in their development, formulation, and market exclusivity. While generic medicines provide cost-effective alternatives to brand-name drugs, super generics offer enhanced efficacy, safety, and value for patients and pharmaceutical companies alike.
As patent protection continues to expire, generic companies must embrace innovation to stay competitive in the healthcare sector. Super generics present a promising avenue for growth, ensuring continued development of advanced pharmaceutical products that improve patient outcomes.
FAQs about Generic Drugs and Super Generics
What does it mean when a drug is generic?
A generic drug is a pharmaceutical product that contains the same active ingredient as a brand-name drug and offers the same therapeutic effect, but at a lower price.
What is an example of a generic drug?
Regular drugs are often brand-name medicines with patent protection, while generic drugs are sold after the patent expires, offering the same active ingredient at a reduced cost.
What is an example of a generic drug?
A common example is ibuprofen, the generic equivalent of Advil or Motrin.
Is paracetamol a generic name?
Yes, paracetamol is the generic name for brand-name drugs such as Tylenol.
What is a super generic?
A super generic is an improved version of a generic drug, offering enhanced efficacy, safety, or drug delivery mechanisms.
What is the meaning of supergeneric?
Supergeneric refers to a modified generic product that provides patients with a better therapeutic effect through advanced formulations.