Table of Contents
When it comes to understanding prescription drugs, the distinction between a brand name drug and a generic drug is crucial. This guide will break down the core of the generic vs brand name drugs debate, focusing on generic names, brand names, active ingredients, and how these factors affect cost, access, and therapeutic effect.
What is a Generic Drug?
A generic drug is a prescription drug marketed under its generic name rather than a trademarked brand name. The generic name reflects the active ingredient that produces the therapeutic effect.
In the United States, once a drug patent expires, other drug manufacturers are allowed to create generic versions of the medication. These must go through the FDA approval process (Food and Drug Administration) to demonstrate bioequivalence—meaning the same dosage form, safety, and therapeutic effect as the original brand name drug.
Although inactive ingredients such as fillers or dyes may differ, the generic medication must match the name brand product in quality, effectiveness, and safety/efficacy. Because they avoid the heavy research and development costs and clinical trials that brand drugs require, generic medicines are typically sold at a lower cost, making them vital to the health care system.
What is a Brand Name Drug (or Non-Generic Drug)?
A brand name drug (sometimes called an original or non-generic drug) is developed, tested, and marketed by a pharmaceutical company under a proprietary trademark. These brand name medications are protected by patent protection, which prevents other drug companies from making a generic version for a set time.
Because drug companies invest heavily in clinical trials, marketing, and research development, the cost of brand drugs is often much higher than their generic counterparts. However, the Food and Drug Administration ensures that both brand name products and their generic versions deliver the same therapeutic results.
Generic vs Brand Name Drugs: Some Keys Similarities
Despite different names and appearances, generic drugs and brand name drugs share far more in common than most people realize. These similarities ensure that switching from a brand to a generic does not compromise safety, quality, or effectiveness.
- Active Ingredient
Both contain the same chemical substance that produces the therapeutic effect, ensuring the medication treats the condition in the same way. - Strength
The dosage amount of the active ingredient is identical, meaning a 20mg generic tablet delivers the same potency as a 20mg brand name tablet. - Dosage Form
Generics must match the brand in form — whether as a tablet, capsule, liquid, or injection — so patients receive the drug in the same format. - Route of Administration
The way the drug enters the body (oral, topical, inhaled, or injected) must be the same, guaranteeing consistent absorption and delivery. - Intended Use
Both versions are approved to treat the same conditions, symptoms, or diseases, so their purpose is identical. - Safety and Effectiveness
The FDA requires generics to be just as safe and effective as their brand name counterparts, ensuring equal protection for patients. - Quality
Manufacturing standards for generics are as strict as for brand name products, covering purity, stability, and consistency. - Bioequivalence
Generics must demonstrate that they work in the body the same way as the brand, releasing the same amount of active ingredient within the same time frame.
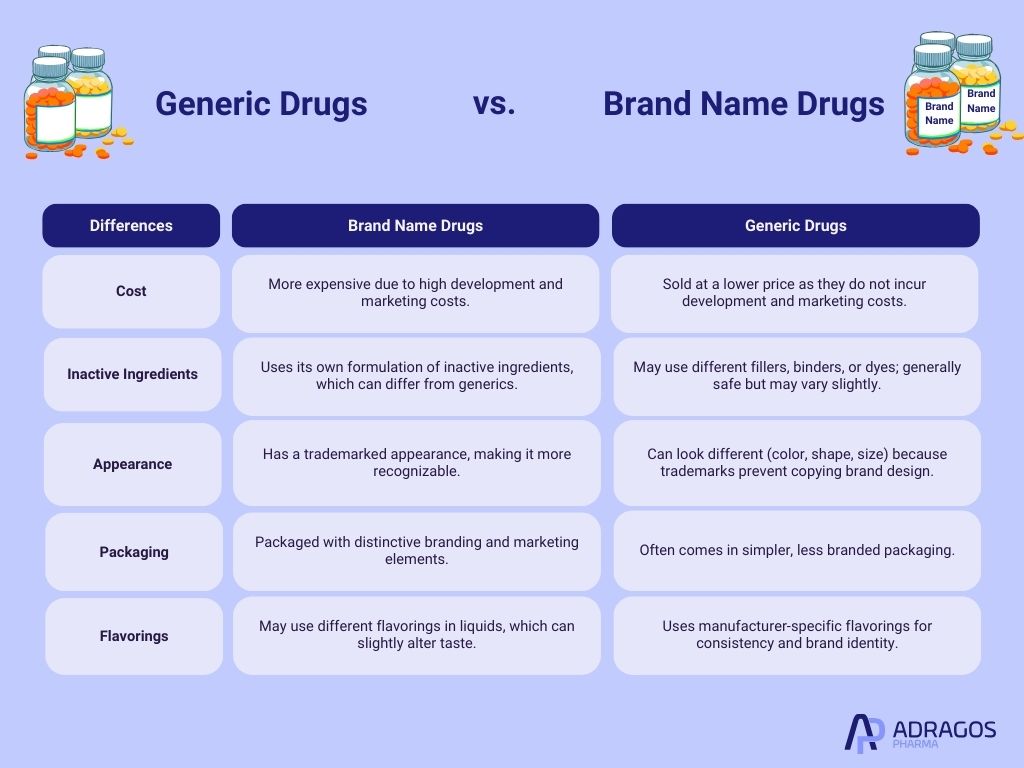
Generic vs Brand Name Drugs: Some Keys Differences
While generics and brands are therapeutically equivalent, there are some important distinctions to be aware of. These differences mainly relate to cost, appearance, and certain non-active components, which can influence patient experience but not the overall effectiveness of the drug.
- Cost
Generics are typically much less expensive because they skip the original research, development, and marketing costs associated with brand drugs. - Inactive Ingredients
While the active ingredient is identical, the non-active ingredients—like fillers, binders, or dyes—can differ. These do not affect the drug’s effectiveness but may impact tolerability in rare cases (e.g., allergies). - Appearance
Generics often differ in color, shape, or size because trademark laws prevent them from copying the exact look of the brand product. - Packaging
Brand name drugs usually have more recognizable or branded packaging, while generics may use simpler designs. - Flavorings
In liquid forms especially, generics may use different flavorings, which can slightly change taste without affecting the therapeutic effect.
The Importance of Generic Name Drugs
Generic medicines ensure affordable access to prescription drugs without sacrificing safety or quality. For patients managing chronic illnesses such as hypertension or diabetes, long-term access to generic medications can mean the difference between consistent care and interrupted treatment.
Pharmacists, Medical Doctors (MDs), Physical Therapist (PTs), and Doctors of Physical Therapy (DPTs) frequently guide patients toward generic drugs as cost-effective alternatives. This not only reduces health care expenses but also helps patients avoid penalties in insurance or Medicare coverage where generic brand choices are encouraged.
How Do Pharmaceutical Companies Handle Generic Name Drugs
When a drug patent expires, drug manufacturers can apply to produce generic versions of the original medication. These must pass rigorous FDA generic drug testing to confirm bioequivalence.
Some pharmaceutical companies specialize in branded generics or niche generic brand medications to serve specific markets. Others diversify into generic drug manufacturing for widespread conditions, supporting patient access and lowering health care system costs.
The Role of Patents in Drug Development
Patent protection allows drug companies to recover investments in research and development. During the patent period, companies have the exclusive right to produce and market the name brand drug. Once that period ends, generic manufacturers can enter the market, often leading to significant cost reductions and increased access for patients.
Generic Medicine Meaning: A Broader Look
The meaning of generic medicine extends beyond just affordability. It ensures equitable health care access while maintaining the same safety/efficacy standards required by the Food and Drug Administration.
Even drugs with a narrow therapeutic index—where small dose changes may impact treatment—must demonstrate consistency through the FDA approval process. This ensures patients receive reliable generic medications comparable to the brand name prescription.
The ongoing debate around generic vs brand name drugs highlights a balance between innovation and accessibility. While brand generic options bring new treatments to the market through innovation, generic drugs ensure that those treatments remain accessible once the drug patent expires.
Whether you’re a healthcare provider, a patient, or a policymaker, understanding the difference between generic and brand name drugs helps ensure informed decisions about care, cost, and quality. Thanks to regulatory oversight by the Food and Drug Administration, both generic medications and brand name drugs meet rigorous safety and efficacy standards, ensuring you receive the best care—regardless of the name on the bottle.
FAQs about Generic vs Brand Name Drugs
Is it better to buy name brand or generic?
Both generic and branded medicines are equally effective because they contain the same active ingredient and must meet the same safety, quality, and efficacy standards set by regulatory agencies like the FDA (Food and Drug Administration).
The main difference is cost: generic drugs are usually much cheaper because they don’t carry the high development, research, and marketing expenses of branded drugs. In most cases, a generic drug works just as well as its brand-name counterpart.
What are the disadvantages of generic drugs?
While generic drugs are highly effective and affordable, they do come with some potential limitations:
Narrow therapeutic index (NTI): For drugs where even small dosage changes can have major effects (like anti-seizure or blood-thinning medications), switching between brand and generic may require extra monitoring by a doctor.
Inactive ingredient differences: Though the active ingredient is the same, fillers, dyes, or preservatives may vary, which can affect people with sensitivities or allergies.
Different appearance: Generic medications may look different in color, shape, or size compared to brand-name drugs, which can be confusing to some patients.
Public perception: Some people wrongly assume that generics are lower quality, even though FDA-approved generics must meet strict standards.
What is the difference between brand name and generic drugs?
Here’s a simple breakdown of the key differences between generic and brand name drugs:
| Feature | Brand Name Drugs | Generic Drugs |
|---|---|---|
| Name | Trade/commercial name (e.g., Lipitor) | Generic/chemical name (e.g., atorvastatin) |
| Cost | Higher | Lower |
| Active ingredient | Same | Same |
| Inactive ingredients | May vary | May vary |
| Patent protection | Yes (for a limited time) | No (after patent expires) |
| Manufacturer | Original pharmaceutical company | Multiple manufacturers |
| FDA Approval | Required | Required |
Both types of prescription drugs must provide the same therapeutic effect, dosage form, and safety.
Why don’t generic drugs work?
In most cases, generic drugs do work just as well as brand-name drugs. If it seems like a generic isn’t working, possible reasons include:
- Patient expectations or psychological factors (known as the placebo/nocebo effect).
- Differences in inactive ingredients, which may affect how the drug is tolerated by some individuals, but not its overall effectiveness.
- Incorrect usage of the medication (e.g., not taking it as prescribed).
- Changes in the patient’s condition, unrelated to the medication.
If you believe a generic medicine isn’t working for you, it’s important to talk to your doctor (MD) or pharmacist, who can help determine whether an adjustment or brand switch is needed.
Are generic drugs lower quality?
No, generic drugs are not lower in quality than brand-name drugs. In fact, in the United States, all generic medications must meet strict standards set by the FDA (Food and Drug Administration). This includes:
- Having the same active ingredient, strength, dosage form, and route of administration as the brand-name drug.
- Demonstrating bioequivalence, meaning they work the same in the body.
- Meeting requirements for manufacturing quality, labeling, and stability.
Generic drugs may have different inactive ingredients (like coloring or preservatives), but this does not affect their effectiveness. If a generic drug is FDA-approved, it is just as safe, effective, and high quality as the brand-name version.
What is the problem with generic medicine?
While generic medicine is highly beneficial, there are a few potential challenges to be aware of:
Global differences: In countries with less regulation than the U.S., the quality of generics may vary. However, FDA-approved generics are rigorously controlled.
Inactive ingredient sensitivity: Some people may react to different fillers, dyes, or preservatives used in generics, even though the active ingredient is the same.
Appearance differences: Generics may look different from brand-name drugs, which can lead to confusion or doubt about effectiveness.
Narrow therapeutic index drugs: For certain medications (like anti-epileptics or thyroid meds), even small changes in blood levels can affect outcomes. Switching from brand to generic may require extra monitoring.
Perception and trust: Some patients believe generics are inferior simply because they cost less or come in different packaging. This is a psychological issue, not a scientific one.
