Table of Contents
Lyophilization, often called freeze drying, has become an indispensable technology in the pharmaceutical industry. But what is lyophilization exactly, and why do pharmaceutical companies depend on it to safeguard their pharmaceutical and biological products? This blog post presents a deep dive into the lyophilization process, including its definition, advantages, disadvantages, function, stages, and the science behind each step. Whether a pharmaceutical beginner or an expert, you’ll gain a comprehensive understanding of the freeze drying process and its many facets.
What is Lyophilization? A Definition
Lyophilization is a sophisticated low temperature dehydration process used to remove water from sensitive materials by freezing them and then sublimating the ice under low pressure. The process occurs at pressures much lower than atmospheric pressure, which is essential for sublimation to take place. Unlike traditional drying methods, freeze drying rapidly converts water from the solid phase directly to the vapor phase, bypassing the liquid phase, through a process called sublimation. Sublimation is driven by differences in vapor pressure between the ice and the surrounding environment. This technique preserves the material’s physical form, activity, and structure by targeting water molecules, which are removed from the material during lyophilization.
The lyophilization process is particularly crucial for pharmaceutical and biological products that are sensitive to higher temperatures. This process ensures that biologically active ingredients remain stable and potent throughout manufacturing, shipping, and storage.
Key Concepts in the Lyophilization and Freeze Drying Process
The freeze drying process involves a precise sequence of well-controlled steps. Key parameters such as shelf temperature, chamber pressure, and eutectic temperature must be carefully monitored for optimal results. Special equipment like a vacuum pump, refrigeration system, shelf fluid (often silicone oil), and accurate product temperature sensors are integral to achieve both primary drying and secondary drying phases.
The aim is to maintain the integrity of biological materials and extend the shelf life of the final product, which may include vaccines, injectable drugs, or other sensitive dosage forms such as tablets, wafers, powders, pellets, and injectables produced by lyophilization. By producing a freeze dried material with a porous structure, the process allows for rapid and easy rehydration at the point of use. Freeze dried products offer advantages such as improved shelf stability, retention of nutritional and organoleptic qualities, and suitability for specialized applications.
Careful process control and optimization are essential to ensure consistent results. Optimizing freeze drying cycles, including both primary and secondary drying cycles, is crucial for achieving desired product quality, efficiency, and stability.
What Makes It Essential and Difficult in Lyophilization
Lyophilization is widely used in pharmaceutical manufacturing due to its exceptional ability to preserve drug efficacy, stabilize sensitive products, and extend shelf life. Despite these significant benefits, the process also poses challenges that require careful consideration and expert control.
Advantages of lyophilization:
- Superior preservation of drug efficacy and quality
- Extended shelf life for sensitive products
- Maintains the stability and structure of delicate drug substances
- Allows for easy and safe reconstitution before use
- Facilitates transport and long-term storage of pharmaceuticals
Disadvantages of lyophilization:
- May complicate reconstitution if the process is not well controlled
- The cycle is considerably longer than traditional drying methods
- High capital investment and operational costs are required for specialized equipment
- Process control is critical, improper optimization can reduce product activity or damage structure
- Risks of instability or degradation if not carefully monitored
- Potential formation of large ice crystals during slow freezing, which can harm product quality.
Is Lyophilization Healthy? Impact on Pharmaceuticals
For the pharmaceutical industry, a key question is whether freeze drying affects the safety and effectiveness of products. Scientific evidence and quality control standards show that lyophilization is a safe process. By maintaining low temperature during the freezing phase and subsequent primary drying sublimation, the original activity and stability of most active components are preserved.
Unlike some drying methods that may use harmful chemicals or excessively high temperatures, freeze drying uses physical means, namely cold, vacuum pump action, and controlled heat transfer, to gently dehydrate the product. The result is a freeze dried product that can safely and easily be reconstituted without compromising patient health or the drug’s efficacy.
The Freeze Drying Method: How Lyophilization Works
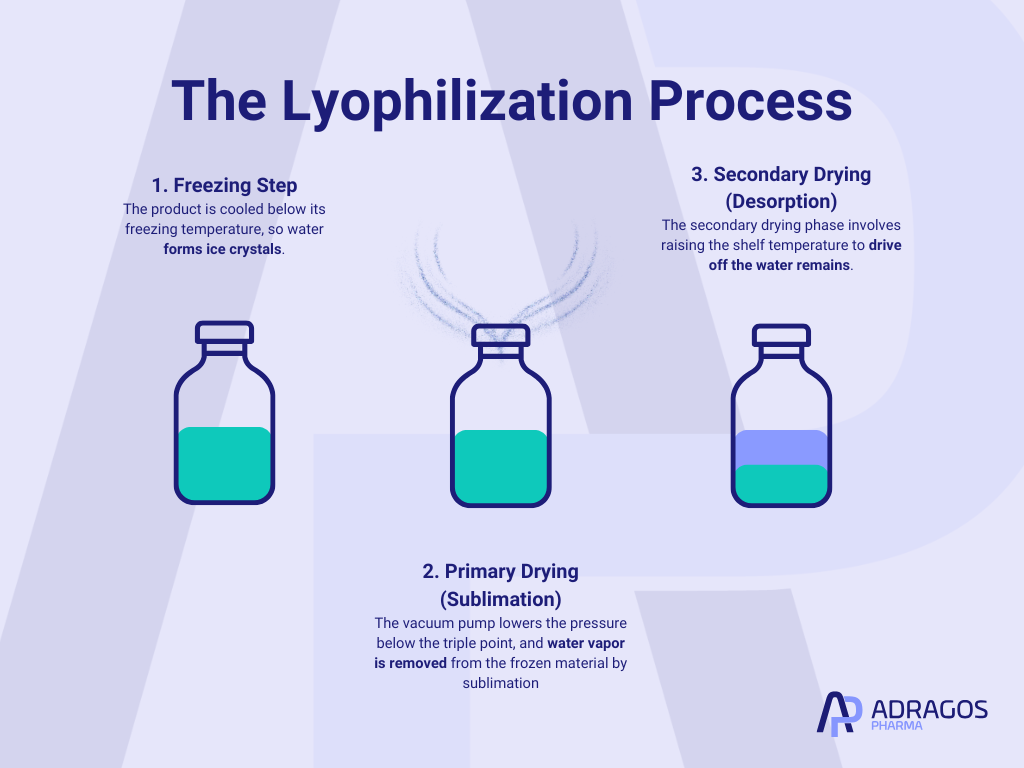
The lyophilization process is characterized by three main steps:
A. Freezing Step
The freezing process is the initial and critical step in lyophilization. The product is cooled below its freezing temperature (often with liquid nitrogen) so water forms ice crystals. This freezing stage determines the size and distribution of ice crystals, which impacts product stability and the efficiency of the drying cycle. Ice formation is influenced by the rate of cooling and atmospheric pressure conditions during the freezing process. Both slow freezing and rapid freezing methods can be used; slow freezing often results in large ice crystals. Manipulating atmospheric pressure during the freezing process can influence ice formation and the structure of the frozen product. During this stage, water within the product matrix becomes frozen water, which will be removed during subsequent drying. Monitoring of eutectic temperature and freezing phase is crucial to avoid unwanted transitions.
B. Primary Drying (Sublimation)
At reduced chamber pressure and controlled shelf temperature, the primary drying phase begins. Here, the vacuum pump lowers the pressure below the triple point, and water vapor is removed from the frozen material by sublimation. Fine precise control over parameters such as product temperature and shelf fluid temperature, often managed with silicone oil, ensures that only the ice (not the solid matrix) is removed. Successful primary drying yields up to 95% water removal.
C. Secondary Drying (Desorption)
After sublimation, some water remains adsorbed. The secondary drying phase involves raising the shelf temperature (still under low pressure) to drive off this bound water. This is critical for achieving optimal shelf life and product stability.
Temperature Control in Lyophilization
Temperature control is fundamental to the success of the lyophilization process, directly impacting the quality, stability, and efficacy of freeze dried pharmaceuticals. During the freeze drying process, the product is first cooled below its triple point to ensure complete solidification. In the primary drying phase, the shelf temperature is carefully maintained, typically between -30°C and -50°C, to promote sublimation without exceeding the product’s eutectic temperature. The condenser temperature is set even lower, often between -60°C and -80°C, to efficiently capture water vapor as it leaves the frozen material.
Precise control of product temperature is essential to prevent melt back or collapse, which can occur if the product is exposed to too much heat. Advanced temperature control systems, sometimes utilizing liquid nitrogen or other cryogenic fluids, enable rapid freezing and maintain the low temperature required throughout the process. This careful management ensures that both primary drying and secondary drying phases proceed efficiently, preserving the porous structure and potency of the final product. By keeping the product temperature below critical thresholds—such as the eutectic point—manufacturers safeguard the integrity of sensitive pharmaceutical products during every stage of the drying process.
Refrigeration and Equipment Used in Lyophilization
The effectiveness of the freeze drying process relies heavily on specialized equipment designed for precise control of temperature and pressure. At the heart of any freeze dryer is a robust refrigeration system, which cools both the condenser and the shelf fluid to the low temperatures necessary for successful sublimation. The condenser temperature typically operates between -60°C and -80°C, efficiently trapping water vapor, while the shelf fluid, often silicone oil, maintains the shelves at -30°C to -50°C to keep the product frozen during primary drying.
A powerful vacuum system is also essential, creating a low pressure environment (usually between 50-200 mTorr) within the vacuum chamber. This reduced pressure enables the direct transition of ice from the solid phase to vapor phase, bypassing the liquid phase and ensuring gentle dehydration of the frozen water in the product. The freeze dryer’s control system allows for precise adjustment of all parameters, including shelf temperature, chamber pressure, and drying time, ensuring optimal results for each batch. Together, these components provide the precise control needed to produce high-quality freeze dried materials in the pharmaceutical industry.
How Long Does Lyophilization Take?
The length of the freeze drying process varies based on product type, chamber pressure, and desired moisture level. The number and duration of freeze drying cycles, including both primary and secondary drying cycles, can significantly impact the total processing time and product quality. Most lyophilization cycles take from several to many hours, sometimes even days, depending on freezing step, primary drying, and secondary drying duration. Rapid heat transfer and efficient vacuum pump operation facilitate shorter cycles, but too much heat can damage the product. Hence, precise control of product temperature, shelf temperature, and eutectic temperature is key.
Why Do Pharmaceutical Companies Rely on Lyophilization?
The ability to extend shelf life, maintain the physical form, and provide products in convenient final containers makes lyophilization invaluable for the pharmaceutical industry. Pharmaceutical applications that require long-term stability at room temperature, rapid dissolution, and preserved potency benefit most from this technology. Lyophilized formulations safeguard labile products such as vaccines, blood plasma, and biologics, serving both commercial and emergency needs.
The Function and Importance of Lyophilization
The main function of freeze drying is to offer unmatched stability for temperature-sensitive biological materials and pharmaceutical products. By removing water while preserving a stable physical form and active structure, the lyophilization process ensures quality control for sensitive dosage forms. This results in pharmaceutical products that have a significantly extended shelf life, easier transport logistics, and lower risk of degradation.
Science and Optimization in the Freeze Drying Process
Process optimization plays a vital role in every lyophilization cycle. Specialists monitor parameters like eutectic point, condenser temperature, shelf fluid flow, vacuum system performance, and triple point stability. Advanced PAT (Process Analytical Technology) tools, including infrared radiation thermography, help finely tune drying kinetics, ensuring reliable product outcomes batch after batch. This ongoing optimization is essential to minimize risks of too much heat exposure, large ice crystals formation, and inefficacies in primary and secondary drying. Optimizing freeze drying cycles, including primary and secondary drying cycles and parameters such as shelf temperature, is crucial not only in pharmaceuticals but also in other industries such as the food industry.
Lyophilization—the gold standard in freeze drying—has transformed the pharmaceutical industry by enabling safe, effective, and long-lasting pharmaceutical and biological products. The food industry also uses freeze drying to preserve high-value products like seasonal fruits, vegetables, instant soups, and coffee, extending shelf-life and maintaining quality. With stringent quality control and a deep understanding of the complex lyophilization process, pharmaceutical companies can deliver innovative, life-saving therapies worldwide. A robust command of parameters like eutectic temperature, primary drying phase, and secondary drying is imperative for optimizing every lyophilization cycle and ensuring the best patient outcomes. After lyophilization, an inert gas such as nitrogen is often used to break the vacuum and preserve product sterility.
Strategic Benefits of Working with a CDMO Partner
Partnering with a contract development and manufacturing organization (CDMO) offers pharmaceutical companies significant strategic advantages in the development and production of lyophilized products. CDMOs bring specialized expertise in formulation development, process optimization, and scale-up, ensuring that each step of the freeze drying process is executed with precise control and efficiency. Their access to advanced freeze drying equipment and analytical instrumentation allows for rigorous quality control and compliance with regulatory standards such as Good Manufacturing Practice (GMP).
Swiss Excellence in Lyophilization: Our Jura Facility at Your Service
Located in the heart of Switzerland, our Jura facility combines over 20 years of specialized expertise with GMP and FDA certification to offer exceptional lyophilization and aseptic fill-finish services for (bio)pharmaceutical products. We support both clinical and commercial manufacturing, with a strong focus on small and flexible batch production, and no minimum batch size requirements. Our lyophilization capabilities include everything from process scale-up and technology transfer to robust project management, aseptic process simulation (APS), and comprehensive stability studies. This ensures your sensitive formulations preserve their activity, potency, and structure at every stage, whether at pilot, clinical, or commercial scale. Our skilled team optimizes each freeze drying cycle and provides dedicated regulatory support, enabling efficient transitions from development to GMP storage and global supply. By integrating cutting-edge technology, meticulous quality control, and tailor-made solutions, our Jura facility stands out as your trusted partner for high-quality, reliable, and fully compliant lyophilized product manufacturing—empowering your success at every step of pharmaceutical innovation.
By leveraging a CDMO’s experience and resources, pharmaceutical companies can accelerate product development timelines, extend shelf life, reduce capital investment in specialized equipment like vacuum systems, and focus on their core strengths such as drug discovery and commercialization. CDMOs also help navigate complex regulatory requirements, ensuring that freeze dried pharmaceuticals are developed and manufactured to the highest standards of quality and efficacy. This collaborative approach supports successful product launches, robust lyophilization cycles, and long-term market success in the pharmaceutical industry.
FAQs about Lyophilization and Freeze Drying
What is lyophilization and why is it used?
Lyophilization, or freeze drying, is a low temperature dehydration process used to preserve sensitive pharmaceutical and biological products. It works by freezing the product and sublimating ice under low pressure, effectively removing water while maintaining product stability and efficacy. This process increases shelf life and maintains potency during storage and transport.
Why are drugs lyophilized?
Many drugs are lyophilized to extend shelf life, stabilize dosage forms, and protect biological materials from degradation during transportation and storage. It is especially crucial for labile injectables, vaccines, and certain antibiotics.
What are the disadvantages of lyophilization?
The main disadvantages are long lyophilization cycle times, high capital and operational costs, risk of too much heat or improper heat transfer damaging sensitive products, and possible formation of large ice crystals that may affect the material’s cell walls and porous structure. Stringent quality control is essential to overcome these limitations.
What are the 4 steps of freeze-drying?
The freeze drying process includes:
Stoppering/final containers: Sealing the freeze dried product to maintain stability and maximize shelf
Freezing step: Cooling the product below its freezing temperature to form ice.
Primary drying phase: Subliming ice under vacuum pump at carefully controlled shelf temperature.
Secondary drying: Removing adsorbed water by increasing temperature under continued low pressure.
What temperature is lyophilization?
Lyophilization (freeze-drying) typically occurs at temperatures between -40°C and -80°C during the initial freezing stage.
- Secondary drying (desorption) stage: The temperature is increased further (often 0°C to +30°C, sometimes up to +40°C) to remove residual moisture.
- Freezing stage: The product is frozen at very low temperatures, usually between -40°C and -80°C.
- Primary drying (sublimation) stage: While under vacuum, heat is gradually applied to raise the temperature, but it generally remains below 0°C (often from -20°C to -5°C) to ensure sublimation of ice.
