In the Japanese pharmaceutical market, a Marketing Authorization Holder (MAH) is legally required to commercialize pharmaceutical products. However, companies often face different regulatory and logistical situations regarding their MAH status. This chapter outlines two specific MAH transfer scenarios and demonstrates how Adragos Pharma can support companies in both cases.
MAH Transfer – MAH Available in Japan
In this scenario, Company A is a European-based pharmaceutical company with both its headquarters and manufacturing sites located in Europe. It already has an established MAH entity in Japan, which allows it to market and distribute products domestically. At some point, Company A decides to transfer its MAH responsibilities to Company B, which is headquartered in the United States. Like Company A, Company B also has a manufacturing site in Europe and a registered MAH entity in Japan.
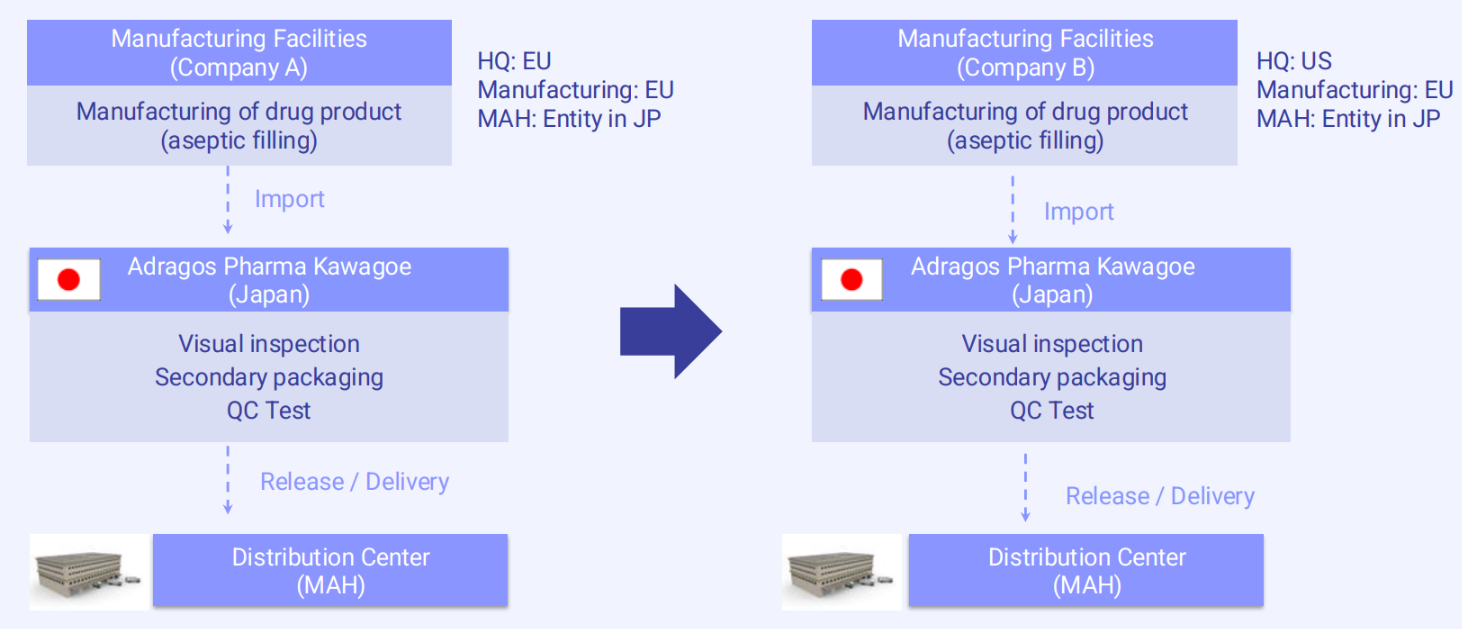
Because both companies have MAH status in Japan and the manufacturing sites remain unchanged including those responsible for filling, visual inspection, and packaging in Europe the transfer process is relatively straightforward. No adjustments to the Japanese supply chain or manufacturing operations are needed.
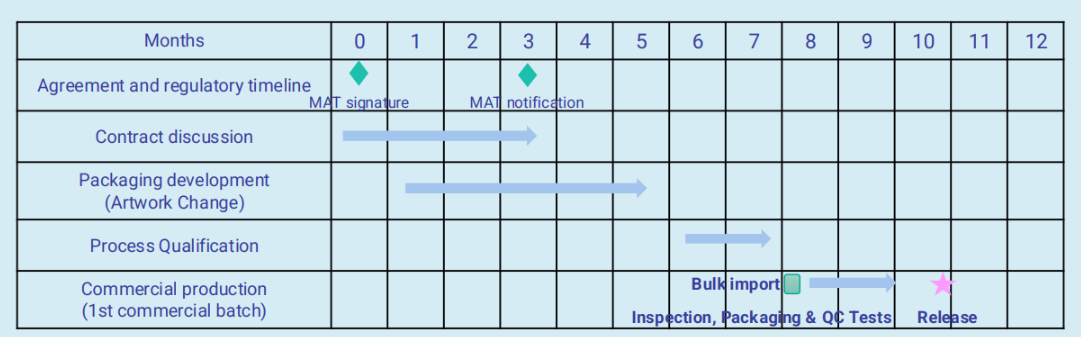
As a result, the timeline for launching the product after initiating the MAH transfer can be significantly shortened. Typically, the transition from the date of the MAH transfer signature to the release of the product can be completed within approximately ten months.
Adragos Pharma plays a central role during this transition. All contractual discussions, including those related to packaging and supply chain, are conducted in English removing the need for Japanese translation and allowing for more efficient communication. Additionally, during the transition period, special solutions such as a dual-barcode system can be introduced.
This allows packaging materials to display both the previous and new MAH information, which is especially useful if there is existing stock of bulk materials. These coordinated efforts ensure a smooth and compliant transition with minimal impact on timelines or supply continuity.
MAH Transfer – MAH Not Available in Japan
A different scenario occurs when the company intending to become the MAH has not yet established a legal MAH entity in Japan. In this case, Company A is again headquartered in Europe and wishes to market its product in Japan but lacks the necessary local MAH status. To address this, it partners with Company B, a UK-based firm that is already registered as an MAH in Japan.
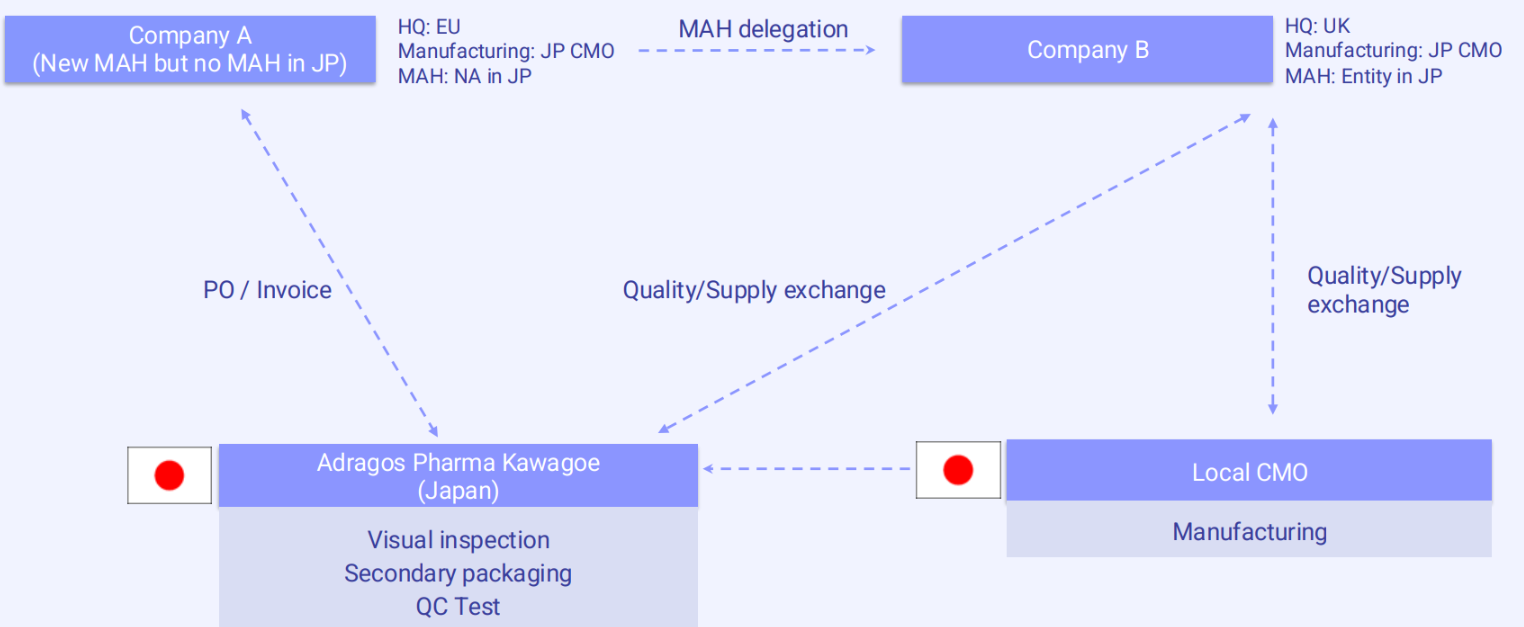
Under this arrangement, Company A remains the main point of contact for all commercial matters, such as purchase orders and invoicing. Meanwhile, Company B, as the registered MAH, is responsible for handling technical, regulatory, quality, and supply related activities within Japan. Adragos Pharma facilitates communication and coordination between both companies, ensuring alignment across all aspects of the transfer and market preparation.
In many cases, three-party meetings are conducted to address the complexities of the handover and establish clear responsibilities. This collaborative approach enables Company A to enter the Japanese market without needing to set up its own MAH entity, significantly reducing the time and investment typically required for market entry.
Moreover, if Company A has not yet identified a suitable MAH partner like Company B, Adragos Pharma can support the search by introducing qualified candidates who meet regulatory requirements and can act as the MAH on Company A’s behalf.
Through expert project management, multilingual communication support, and regulatory insight, Adragos Pharma ensures that companies regardless of their MAH status in Japan can successfully transfer marketing authorization and bring their products to the Japanese market efficiently and compliantly.

