Sterile manufacturing is essential for ensuring the safety and efficacy of drug products, especially sterile injectables. With the growing demand for sterile products, the need for precise, contamination-free manufacturing processes has never been more critical.
What is Sterile Manufacturing?
Sterile manufacturing involves the production of pharmaceuticals in an environment free from viable microorganisms. This rigorous process is vital for maintaining the integrity of drug products, particularly those administered via injection, where even the smallest microbial contamination can lead to severe health risks.
Key Processes in Sterile Manufacturing
Aseptic Filling Process
Aseptic manufacturing is a highly controlled process where drug products are filled into sterile containers without exposing them to contamination. The filling process, a crucial part of aseptic manufacturing, involves the careful handling of sterile products in clean rooms equipped with advanced air filtration systems and stringent environmental controls, ensuring that the products remain sterile throughout.
Terminal Sterilization
Terminal sterilization is a method that applies heat, chemicals, or radiation to sealed drug products to eliminate any microorganisms. This process is typically used when the product’s formulation can withstand such treatments, ensuring sterility post-packaging. Terminally sterilized products are often preferred for their higher assurance of sterility compared to aseptic processes.
Clean Room Technology
Clean rooms are essential to sterile manufacturing, providing a controlled environment with minimal levels of airborne particles, microorganisms, and chemical vapors. These rooms are maintained through advanced air filtration, clean steam systems, and stringent operational procedures to ensure that the products manufactured within them are free from contamination.
Products in Sterile Manufacturing
Sterile manufacturing covers a wide range of products, including ampoules, vials, and IV bags.
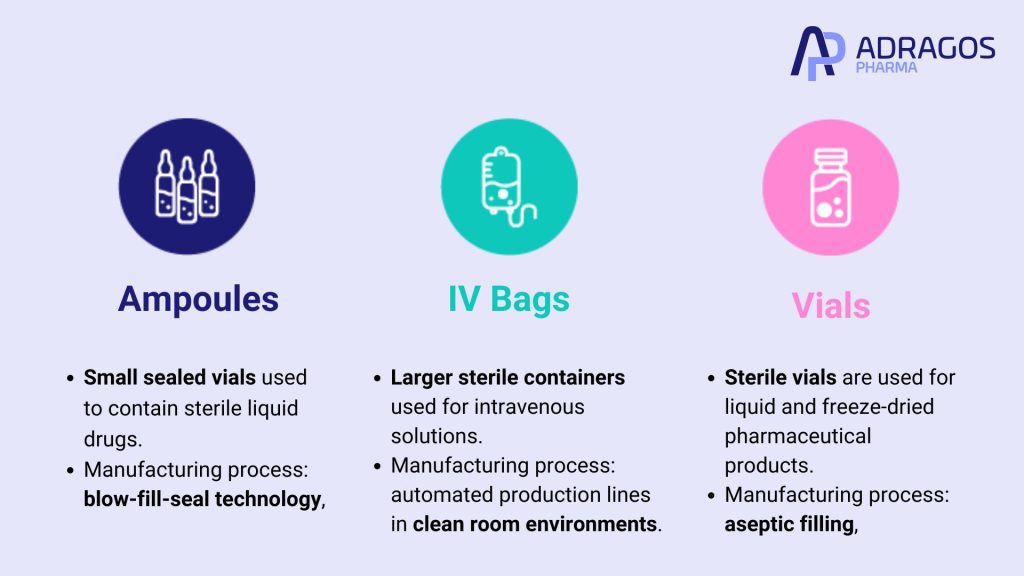
Ampoules
These are small sealed vials used to contain sterile liquid drugs. The manufacturing process for ampoules involves blow-fill-seal technology, which forms, fills, and seals the ampoules in a single automated process, reducing the risk of contamination.
IV Bags
These are larger sterile containers used for intravenous solutions. The manufacturing of IV bags involves automated production lines in clean room environments, ensuring that the solutions remain sterile throughout the process. Common products include saline, glucose, and various medication infusions.
Vials
Sterile vials are used for both liquid and freeze-dried (lyophilized) pharmaceutical products. The process involves careful aseptic filling, followed by either freeze-drying or terminal sterilization, depending on the product’s stability requirements.
Quality Control in Sterile Manufacturing
Quality control is a critical aspect of sterile manufacturing, involving rigorous testing and monitoring to ensure that all products meet the required sterility standards. This includes:
Microbial Testing: To detect any potential contamination, products are tested for the presence of microorganisms at various stages of the manufacturing process.
Particulate Matter Testing: Ensuring that products are free from particulate contamination, which is particularly important for injectables.
Sterility Testing: Final products undergo sterility testing to confirm that they are free from any viable microorganisms before being released for distribution.
Packaging and Sterility
Packaging plays a crucial role in maintaining the sterility of pharmaceutical products. Aseptic packaging ensures that the drug product remains sterile until it reaches the patient. This involves using materials and packaging components that prevent contamination and maintain product integrity throughout its shelf life.
Challenges in Sterile Manufacturing
Sterile manufacturing is fraught with challenges, primarily due to the need for absolute sterility and precision. Some of the key challenges include:
Maintaining Sterile Conditions: Ensuring that the entire manufacturing environment, including clean rooms, equipment, and personnel, remains free from contamination.
Human Intervention: Minimizing human intervention is crucial as it is one of the primary sources of contamination. Automation and advanced technologies are increasingly used to reduce this risk.
Cross Contamination: In multi-product facilities, preventing cross contamination between different products is vital. This requires careful planning, dedicated equipment, and strict procedural controls.
Additionally, manufacturing organizations face significant challenges in building capacity for sterile production lines. These include lengthy installation times for new lines, the current global supply crisis, and complexities in partnering with contract manufacturing organizations to improve capacity amid existing operational inefficiencies.
Innovations in Sterile Manufacturing
The pharmaceutical industry is continuously evolving, with new technologies and processes being developed to improve sterile manufacturing. Some of the recent innovations include:
Advanced Aseptic Processing: Utilizing robotic systems and isolators to reduce human intervention and contamination risks.
Improved Terminal Sterilization Methods: Development of new sterilization techniques that are more effective and less damaging to sensitive drug formulations.
Data-Driven Decision Making: Leveraging big data and advanced analytics to monitor and optimize manufacturing processes in real time, ensuring consistent product quality.
Sterile manufacturing is essential for producing safe and effective pharmaceutical products. By adhering to strict sterility standards and leveraging advanced technologies, manufacturers can ensure that their products meet the highest quality standards. As the demand for sterile products continues to grow, ongoing innovation and rigorous quality control will be key to meeting the pharmaceutical industry’s needs.
FAQs about Sterile Manufacturing
What is an example of sterile manufacturing?
An example is the aseptic fill finish process used for sterile injectable products, where the drug product is filled in a sterile environment to prevent contamination.
What is the difference between sterile and aseptic?
Sterile means completely free from all microorganisms, while aseptic refers to a controlled process that prevents contamination during manufacturing.
What are sterile manufactured drug products?
These are pharmaceuticals produced in an environment free from viable microorganisms, ensuring they are safe for patient use.
What is considered a sterile product?
A sterile product is one that is free from viable microorganisms and meets strict sterility standards required for pharmaceutical use.
