Table of Contents
The pharmaceutical industry thrives on innovation, collaboration, and the strategic exchange of assets and technologies. As companies seek to maximize their commercial potential, minimize development risks, and expand their market reach, out licensing has become an essential component of modern business strategy. Whether you are a small or large pharmaceutical company, understanding out licensing is crucial to making informed decisions and driving company success.
In this comprehensive guide, we will explore the meaning of out licensing, its key aspects, how it differs from in licensing, and practical steps for navigating the out licensing process. We will also highlight how Adragos Pharma’s Athens facility serves as an effective out licensing partner for companies seeking new pharma licensing opportunities.
What is Out Licensing? A Clear Definition
Out licensing is the process by which a company (the licensor) grants certain rights, often related to a drug, technology, or intellectual property, to another company (the licensee) to further develop, manufacture, market, or sell. Instead of commercializing or developing an asset themselves, licensors leverage the resources, expertise, and market reach of their partners. Companies often out license their assets to expand market access and form strategic partnerships, allowing them to enter new geographic regions or benefit from external expertise.
The out licensing model is prevalent among pharmaceutical companies, biotech firms, and technology providers looking to generate revenue, mitigate risks, and enable their assets to realize full commercial opportunity. Licenses are legal permissions granted to external partners, allowing them to develop, manufacture, or commercialize drugs or technologies under agreed terms.
Key Aspects of Out Licensing
Let’s break down the key aspects and benefits that make out licensing a strategic option for companies engaged in the pharmaceutical industry:
1. Revenue Generation
Out licensing enables a company to generate revenue from unused or underdeveloped assets, such as drug candidates, platform technologies, or proprietary processes. Payments can take various forms, including upfront payments, milestone payments, and royalties based on product portfolios performance.
2. Risk Mitigation and Sharing
One of the key aspects of out licensing is risk sharing. As development costs, regulatory approval, and market entry can be resource-intensive and uncertain, licensors can transfer much of the financial risk and development risks to the licensee. This arrangement supports mitigating risks while still capturing potential upside through deal terms like royalties and milestones.
3. Market Expansion
Out licensing opens doors for market expansion, allowing pharmaceutical companies to expand into new markets by leveraging the established market reach of the licensee. This is particularly valuable for entering diverse geographical regions or new therapeutic areas.
4. Strategic Partnerships and Focus
Forming pharma partnership deals through out licensing agreements creates strategic synergies and enables both parties to focus on their core competencies. For example, a biotech firm may specialize in early-stage development, while a larger pharma company can handle late stage trials, regulatory approval, and commercialization.
5. Monetizing Intellectual Property
For companies with valuable but unexploited intellectual property, out licensing is a practical way to unlock its commercial potential and boost company value using multiple valuation methods such as risk adjusted net present value.
Understanding the Difference: Out Licensing vs. In Licensing
While out licensing is about granting rights, in licensing is about acquiring them. Here is a quick summary:
- Out licensing: The company (licensor) grants rights to another party to exploit their technologies or products.
- In licensing: The company (licensee) obtains rights from another party to use their intellectual property or assets.
Both strategies help pharmaceutical companies achieve their strategic goals, such as accessing innovative technologies (via in licensing) or extracting value from their existing pipeline (through out licensing).
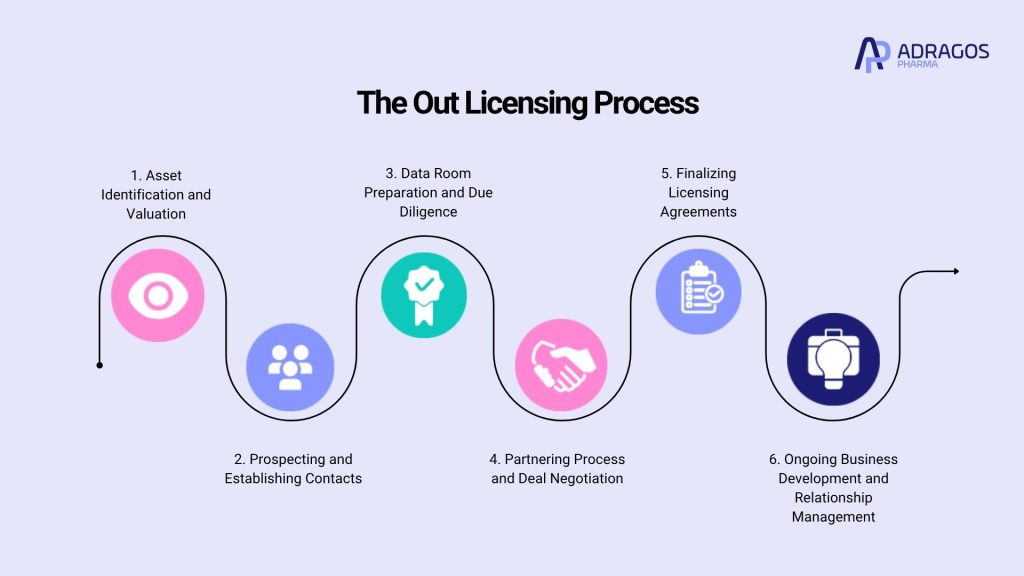
Step-by-Step: The Out Licensing Process
Successfully navigating the out licensing process involves several critical stages. Here’s a practical breakdown:
1. Asset Identification and Valuation
- Companies must identify promising assets, such as a biopharmaceutical asset or proprietary technology in house, for out licensing.
- Accurate asset valuation using multiple valuation methods like risk adjusted net present value is essential to understand the worth of the offering.
2. Prospecting and Establishing Contacts
- Identifying potential partners and prospective partners is a critical step. These could include regional companies, large pharmaceutical firms, or innovative startups.
- Establishing contacts and leveraging networks or platforms is crucial for presenting your asset to suitable potential licensees.
3. Data Room Preparation and Due Diligence
- Preparing a virtual data room or data room, a secure repository for confidential documents, enables prospective partners to perform due diligence on your asset, development, clinical data, and intellectual property status.
4. Partnering Process and Deal Negotiation
- The partnering process involves making introductions, technical meetings, and presenting the asset’s commercial opportunity.
- Negotiating deal terms, such as upfront payments, milestone payments, royalties, and responsibilities for regulatory approval, is central to achieving a mutually beneficial licensing deal.
5. Finalizing Licensing Agreements
- Once terms are settled, both parties sign binding licensing agreements that define rights, obligations, product portfolios, and financial arrangements.
6. Ongoing Business Development and Relationship Management
- Business development teams from both sides continue to collaborate, monitor progress, and adjust to challenges as development and commercialization proceed.
Benefits of Out Licensing for Pharmaceutical Companies
Pharmaceutical companies, irrespective of their scale, can benefit in several ways by adopting an out licensing strategy:
1. Generate Revenue from Underutilized Assets
Vacant technologies and assets can be transformed into reliable income streams, especially when internal development or marketing is not feasible.
2. Reduce Development and Financial Risk
By collaborating with a licensing partner, companies can share or offload the burden of high development costs, regulatory approval hurdles, and other development risks.
3. Accelerate Access to New Markets and Patients
Through market expansion with established external partners, assets are more likely to enter new markets quickly, helping expand company impact and support global health.
4. Focus on Core Strengths and Complementary Capabilities
Companies can focus on their strengths, such as discovery or technology development, while external partners with complementary capabilities handle later development and commercialization.
5. Foster Industry Partnerships and Strategic Synergies
Entering out licensing agreements builds lasting relationships, encourages pharma partnership deals, and drives collective progress within the pharmaceutical industry.
How Adragos Pharma Can Help You Out Licensing
Adragos Pharma’s facility in Athens is dedicated to helping pharmaceutical companies unlock the benefits of out licensing. Here is how our out licensing model empowers our partners:
- Access to Our Generic and Value Added Medicines Product Portfolios: We offer an extensive and growing selection of finished dosage forms ready for global and regional expansion.
- Accelerate Entry into New Markets: Our robust network, regulatory know-how, and established presence help partners effectively expand their market reach.
- Mutually Beneficial Out Licensing Agreements: We utilize a collaborative approach to optimize financial returns
- Dedicated Support from Out Licensing Experts: Our seasoned business development team assists with every aspect of the out licensing process, from data room preparation and due diligence to deal negotiation and ongoing support.
- Shared Mission for Global Health: By partnering with Adragos Pharma, you join us in delivering vital healthcare solutions to more markets, faster.
Out licensing is more than a financial transaction; it is a strategic approach allowing pharmaceutical companies to expand their market presence, focus on core strengths, and build enduring industry partnerships. By leveraging the out licensing process, firms can unlock hidden value, drive innovation, and collaborate for mutual benefit.
Whether you are a startup with groundbreaking technologies or a global leader managing a vast product portfolio, informed decisions about out licensing can accelerate your journey toward success in the ever-evolving pharmaceutical industry.
FAQs About Out Licensing
What is the meaning of out-licensing?
Out licensing refers to the process where a company grants permission to another party (licensee) to use, develop, produce, or commercialize its technologies, products, or intellectual property. This usually involves formal licensing agreements and is a common practice in the pharmaceutical industry to generate revenue and expand market presence.
What is an example of out-licensing?
An example would be a company with a new drug candidate out licensing it to a larger pharmaceutical company that has the resources to conduct late stage trials, navigate regulatory approval, and launch the drug in new markets. In this arrangement, the licensee typically pays upfront payments, milestone payments, and royalties to the original developer.
What is the out-licensing strategy?
The out-licensing strategy is a strategic approach by which a company leverages its own assets or technologies by partnering with licensees who can best maximize their potential. This strategy allows companies to focus on their strengths, mitigate risks, enter new markets efficiently, and foster strong partnerships.
What is the difference between Inlicense and Outlicense?
- In licensing: A company acquires rights from another party to use their intellectual property or products, thus expanding its product portfolios.
- Out licensing: A company grants another party the rights to use or commercialize its own assets or technologies.
Both forms of licensing are used strategically to meet business development goals, access complementary capabilities, and optimize commercial potential.
