Pharmaceutical companies are constantly searching for innovative drug delivery systems to enhance product stability, prolong shelf life, and ensure efficacy. One such technology that has revolutionized the industry is lyophilisation (freeze-drying), which produces lyophilisates—a highly stable, easy-to-reconstitute dosage form.
Lyophilisation plays a crucial role in the development of biologicals, peptides, and sterile formulations, particularly in clinical trials and commercial-scale manufacturing. This article explores what lyophilisate is, its benefits, applications, and its future in the pharmaceutical industry.
What is Lyophilisate?
A lyophilisate is a pharmaceutical product obtained through lyophilisation (freeze-drying), a process that removes water from a substance while maintaining its structure and bioactivity. This technique is commonly used for heat-sensitive drugs, biologics, peptides, and sterile injectables, ensuring their long-term stability.
Lyophilisation is achieved in three key phases:
Freezing – The drug solution is rapidly frozen to convert water into ice.
Primary Drying (Sublimation) – Ice is removed under vacuum conditions.
Secondary Drying (Desorption) – Remaining moisture is eliminated to enhance stability.
The result is a dry, porous cake-like structure, which can be reconstituted by adding a diluent, such as sterile water or saline solution, before administration.
Why is Lyophilisation Important in Pharmaceuticals?
1. Stability & Shelf Life Extension
One of the primary advantages of lyophilisation is its ability to preserve drug stability. Many biopharmaceuticals, vaccines, and peptide-based drugs degrade in liquid form but can maintain efficacy when freeze-dried. The data supporting the stability benefits of lyophilisation is robust, indicating that this process effectively extends the shelf life of these treatments.
2. Ideal for Heat-Sensitive Compounds
Many pharmaceutical compounds, especially biological products, sterile suspensions, and peptides, are sensitive to heat. Lyophilisation ensures drug preservation without thermal degradation.
Lyophilisation is particularly important for heat-sensitive drugs used in children, as it helps maintain the efficacy and safety of medications tailored for young patients.
3. Improved Solubility & Rapid Reconstitution
Lyophilisates dissolve quickly upon reconstitution, making them ideal for emergency treatments and clinical trials where rapid preparation is crucial. The specific characteristics and details of lyophilisates, such as improved solubility and rapid reconstitution, contribute to their effectiveness in these scenarios.
4. Enhanced Patient Safety & Compliance
Lyophilised drugs eliminate the need for complex cold chain logistics, reducing storage and transport costs for patients. This is especially beneficial for vaccines, biologics, and controlled substances.
Lyophilisate Applications in the Pharmaceutical Industry
Lyophilisation technology is widely used across various pharmaceutical sectors. Below are its key applications:
1. Clinical Trials & Drug Development
Lyophilisation is essential for early-stage drug development, particularly for manufacturing clinical batches under GMP/FDA conditions. The ability to process very small bulk solution volumes (200-300 ml) makes it ideal for preclinical and stability studies.
Accurate prescription descriptions for lyophilisates in clinical trials are crucial to ensure proper regulatory compliance and reimbursement.
2. Biologics & Peptide-Based Therapies
Biological drugs, including monoclonal antibodies, vaccines, and peptides, require lyophilisation to maintain structural integrity. These molecules are highly sensitive and need complex formulation techniques to ensure stability.
Lyophilised biologics and peptides are commonly used in adults for various medical treatments.
3. Ophthalmic & Sterile Injectable Products
Ophthalmic drugs and injectable formulations benefit from lyophilisation due to their need for high purity and sterility. Lyophilised vials ensure the sterility of small molecules, diluents, and controlled substances. Healthcare professionals should be aware of the specific requirements for lyophilised ophthalmic and injectable products.
4. Personalized Medicine & Small Batch Production
Modern pharmaceutical trends emphasize personalized medicine. Lyophilisation enables the production of small-scale, customized drug formulations for specific patient needs. This process allows for personalized treatments for patients who need to be treated with specific formulations.
Lyophilisation Expertise at Our Jura Facility
At Adragos Pharma, our Jura (Switzerland) facility specializes in two distinct dosage forms:
Liquid products aseptically filled
Lyophilized products, both filled in vials
Our Capabilities:
Our facility is equipped with state-of-the-art technology to support pharmaceutical companies in clinical development and commercial manufacturing. Our key capabilities include:
✅ Aseptic Filling
✅ Liquid Filling
✅ Complex Formulation
✅ Peptides & Biological Products
✅ Controlled Substances
✅ Small Molecules & Sterile Suspensions
✅ Ophthalmic Products
✅ Placebos & Diluents
✅ Vial Filling & Lyophilisation (3.5 m² Lyophilizer)
With no minimum batch size imposed, we specialize in clinical batch manufacturing from very small bulk solution volumes (200-300 ml), making us an ideal partner for early-stage pharmaceutical development.
We manufacture batches for various pharmaceutical development phases, from GMP and FDA-compliant production (scale-up, preclinical studies, and stability testing) to full clinical trial material manufacturing.
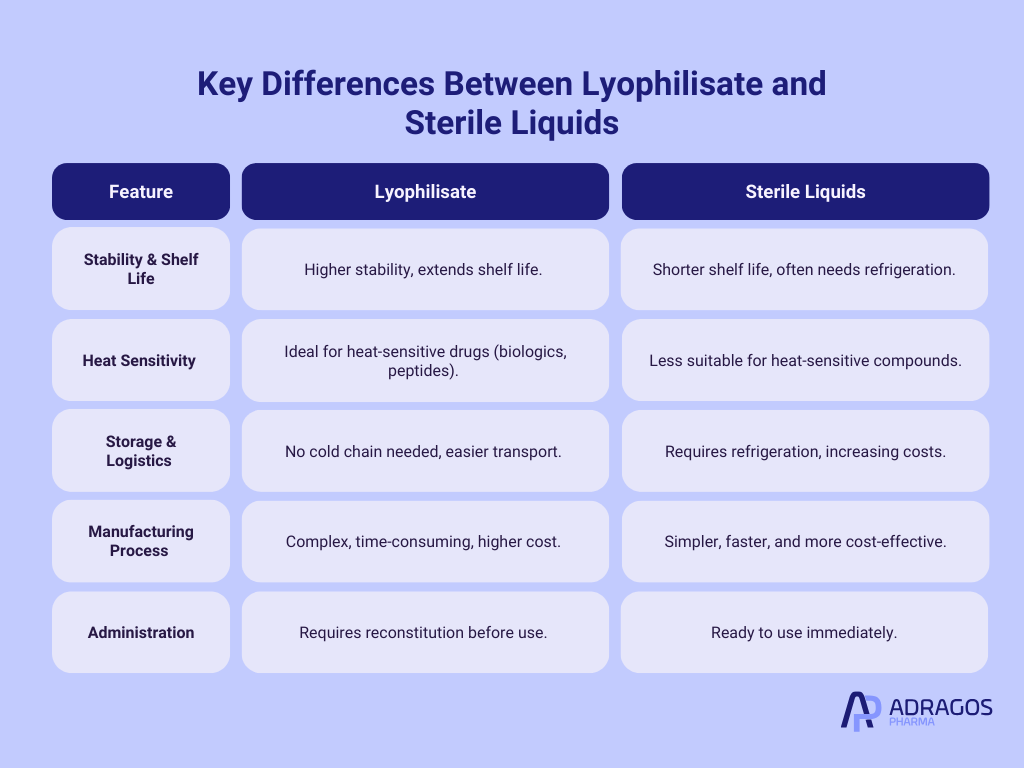
Lyophilisate vs. Sterile Liquids: Key Differences
To better understand the advantages and trade-offs between Lyophilisate and Sterile Liquids, the table below explores their key differences in terms of stability, storage, manufacturing, and usage in pharmaceuticals.
Challenges in Lyophilisation Manufacturing
Despite its advantages, lyophilisation presents several challenges:
High Manufacturing Costs – Freeze-drying requires specialized equipment and controlled conditions.
Complex Formulation Requirements – Peptides, biologics, and sterile suspensions demand precise formulation expertise.
Regulatory Compliance – Lyophilised pharmaceuticals must meet FDA and GMP regulations, ensuring sterility and efficacy.
Time-Intensive Process – The lyophilisation cycle can take hours to days, depending on the product complexity.
Our Jura-based facility at Adragos Pharma is designed to optimize efficiency and compliance, ensuring high-quality lyophilised product manufacturing.
The Future of Lyophilisation in Pharma
The pharmaceutical industry is witnessing advancements in lyophilisation technology aimed at increasing efficiency and reducing costs. Key trends include:
Automated Lyophilisation Systems – Enhancing precision and scalability.
Continuous Lyophilisation – Reducing processing time.
Lyophilised mRNA Vaccines – Supporting global vaccine distribution.
Improved Formulation Technologies – Reducing the need for cold storage.
The future of lyophilisation is innovation-driven, focusing on faster, cost-effective, and patient-centric drug formulations. Lyophilisation technology is transforming pharmaceutical manufacturing, particularly for biologics, peptides, and sterile formulations. Its ability to extend shelf life, enhance drug stability, and support small-batch production makes it indispensable for clinical trials and commercial-scale operations. Additionally, lyophilisation supports comprehensive treatment frameworks that include psychological treatment, addressing both physiological and psychological aspects of patient care.
For pharmaceutical companies, investing in lyophilisation capabilities ensures the ability to develop stable, effective, and patient-friendly drug formulations in an increasingly competitive market.
FAQs about Lyophilisate
What is Lyophilisate?
A lyophilisate is a pharmaceutical product obtained through lyophilisation (freeze-drying) to improve stability and shelf life.
What is the Difference Between Orodispersible Tablets and Lyophilisate?
Lyophilisates require reconstitution for injection, while orodispersible tablets dissolve directly in the mouth without water.
What Are the Side Effects of Lyophilisate?
Lyophilisates themselves do not cause side effects, but the drug contained within them may have specific adverse effects depending on the formulation.
What is a Lyophilised Tablet?
A lyophilised tablet is a freeze-dried oral dosage form designed for fast dissolution, commonly used in orodispersible drugs.