I have always been fascinated by factories, and find the concept of taking individual components and transforming them into finished products truly satisfying. It is fascinating to watch the machines here in Livron filling vials with medicines, ready to treat patients.
Within the Livron facility, a team of about 150 people makes medicines. Some operate the filling lines, while others carry out further vital processes such as visual inspections and final packaging, and staff the warehouse.
The first step of the production process is to make a solution or suspension of the active pharmaceutical ingredient. This is fed into the vials or ampoules, under an atmosphere of nitrogen to prevent contamination with any aerobic microorganisms and to ensure it does not react with oxygen in the air.
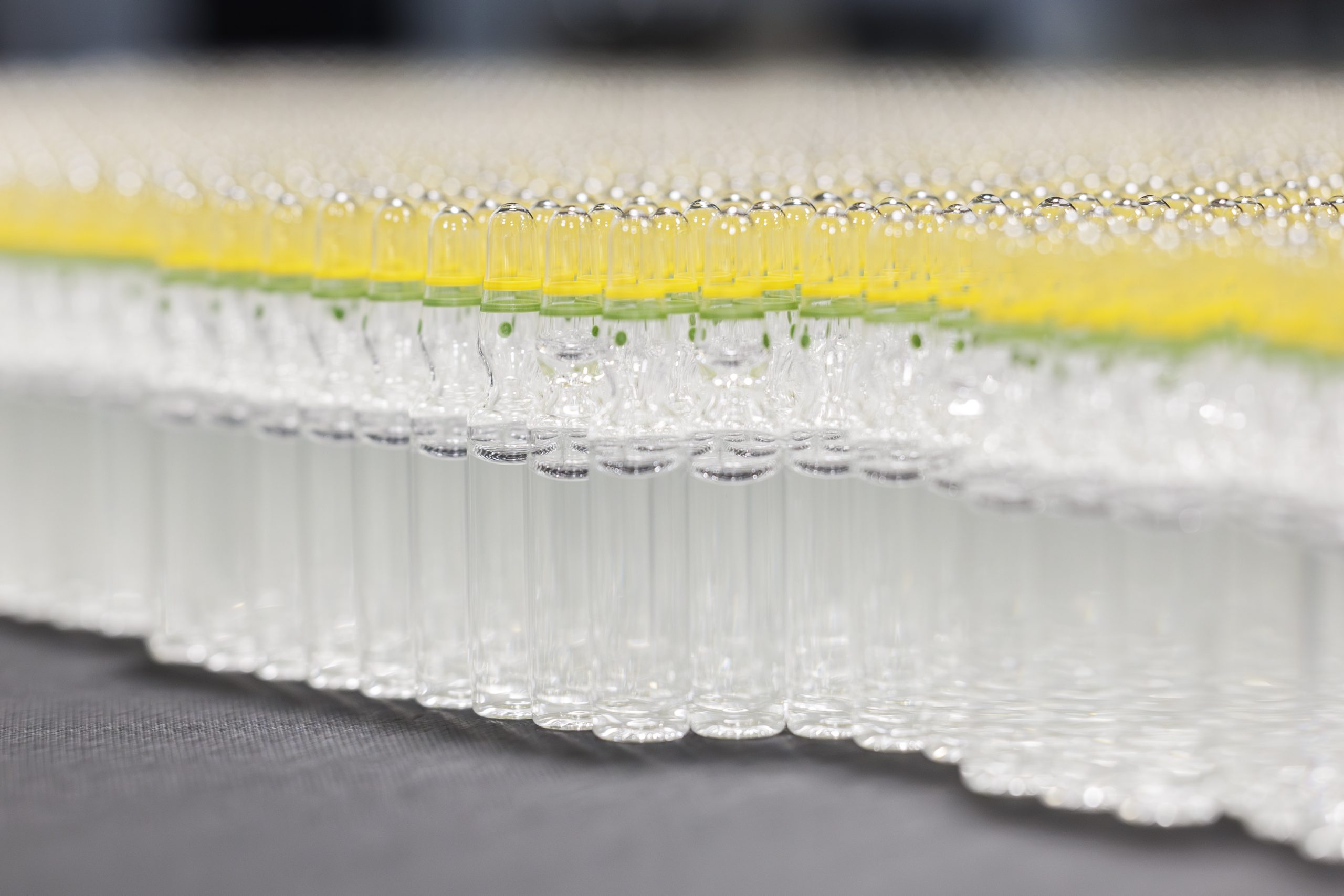
The containers and container closures are all clean when they arrive in our warehouse, but of course, we have to sterilize them as well. The empty vials are placed inside a washing machine, where they are ultrasonically cleaned. We then flush them with a gas (normally air) and then water for injection. They are then fed into a sterilizer, where they pass through at a temperature of 300°C for 20 minutes. This will destroy any residual microbes or endotoxins that could contaminate the product.
All of the filling is carried out in a Class A chamber to ensure sterility, and no human operators are allowed to enter during the filling process. If something goes wrong and human intervention is required, production must stop, and any product that is within the area will have to be discarded before production restarts.
Once the product is closed, there is no risk of contamination, and it will pass through an autoclave for terminal sterilization, if the product is compatible with heat treatment. The next step is a visual inspection, where we look for tiny cracks or flaws in the glass, and then the labels are applied. The last step is the final packaging, where the vials are filled into boxes ready for dispatch.
Wider capabilities
We manufacture suppositories as well as filled glass vials for injection. The process is surprisingly similar – the main difference is that instead of creating a solution or suspension of the drug in water, it is in a wax-like medium that melts when inserted. It is then filled into a suppository mold rather than a vial or ampoule.
We also have a range of special capabilities at the site, such as the ability to work with controlled drugs and highly viscous liquids. The latter typically requires us to slow the line down, reducing the maximum filling capacity. There are cold rooms for products that require refrigeration, and we will carefully log the time the products spend out of the cold room for operations such as final packaging and visual inspection.
The final product must be completely sterile, and this is why a terminal sterilization step is usually included. However, this involves heat treatment, and not all of the molecules we work with are stable at high temperatures.
For these products, we must use aseptic processes throughout to ensure everything remains sterile within the filled product. One way this can be done, if the product is a solution rather than a suspension, is by filtering the solution through filters with small pore sizes to catch any microorganisms that might be present, while still allowing the product to pass through.
Increased capacity
Two new lines are now being installed here in Livron. One, a filling line, is currently in the qualification stage, and we hope it will be fully up and running by the start of 2025. To start with, we will be filling products that will be terminally sterilized on the new lines, as this is quicker to get up and running than aseptic filling. The second new line is for packaging, and the plan is to install this in 2025.
Although they work on the same principles, the new machines are much more controllable than our two older filling lines. For example, on the old machines, the flame had to be adjusted manually, but on the new ones, the gas flow can be controlled electronically. The plan is that the lines will operate in parallel; once the new lines are fully commissioned, we expect the older lines will concentrate on terminal sterilization.

We have hired eight new operators, who have been given training from Syntegon, the filling machine’s manufacturer, and they will be able to carry out multiple functions to maximize our flexibility. The team will be working on a two-shift basis, at least to start with, and thus operational for 16 hours a day. In a single day, once the new line is fully up and running, we will aim to fill 100,000 vials a day, if no product changeover is required, with the lines operational 24 hours a day for five days a week.
One of our aims for 2025 is to work on the changeover, to make it more efficient. We’ve also started a regular meeting, getting all the functions such as quality, IT, and maintenance together every other week, to make everything work more smoothly.
We are also investing heavily in training the operators of the future. Looking back 40 years, it was much easier to recruit people to work within the plant as the operations were generally simpler and required far less training. Now, the complexities of modern pharmaceutical manufacturing mean you cannot simply shift someone from one area to another without investing in giving them the specific skills they require for the new role.
Of course, as a regulated business, it takes time to make changes and improvements in pharmaceutical manufacturing facilities. If you do not respect the rules, there is no business. But by following the rules, the product can be made safely, meeting the needs of both the customer and the patient.
